Determination of Polar Compounds in Used Frying Oils and Fats by Adsorption Chromatography
The Author: Gloria Márquez-Ruiz, Instituto de Ciencia y Tecnología de Alimentos y Nutrición (ICTAN-CSIC), José Antonio Novais, 10, 28040 Madrid, Spain
1. Introduction
During frying, new compounds are formed due to the joint action of high temperature and the presence of air and moisture. The most abundant compounds formed possess higher polarity than their parent triacylglycerols (TG) and low volatility. Hence adsorption chromatography is the most appropriate technique to determine the alteration level in used frying oils and fats by means of isolation and quantification of the total polar compounds. These include modified TG with at least one of the three fatty acyl chains altered, resulting from oxidation and thermal changes taking place in the unsaturated fatty acids, i.e. oxidized monomeric, dimeric and oligomeric TG. Also included are free fatty acids and diacylglycerols released through hydrolytic reactions. Only minor TG containing cyclic or isomeric fatty acids and no oxygenated functions are excluded. Advantages of determination of polar compounds in comparison to other criteria used, such as oxidized fatty acids insoluble in petroleum ether, smoke point and free fatty acids, are mainly the following: (i) values obtained provide a direct measurement of the degradation produced by the different variables involved in the frying process, and (ii) determination is independent of the type of oil used in frying since initial values of polar compounds are similar in unused oils. Most of the present regulations limiting the degradation of used frying fats and oils for human consumption have established a maximum level of polar compounds of around 25% [1].
In this article, the basis of analysis of total polar compounds by adsorption chromatography and gravimetric determination by the Standard IUPAC Method and their modifications will be discussed.
2. Standardized IUPAC Method 2.507
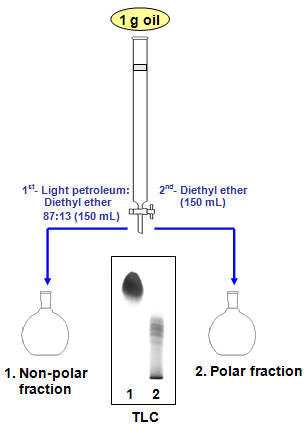
Figure 1 illustrates the analytical method followed to determine total polar compounds according to the Standard IUPAC Method 2.507 [2]. The method was proposed in 1981 by the International Union of Pure and Applied Chemistry [3], three years after publication of the original method [4]. Then, essentially the same procedure was adopted by other official methods [5,6].
Figure 1. Schematic separation of polar compounds by silica column chromatography following the Standard IUPAC Method 2.507.
According to the Standard IUPAC Method, 1 g of used frying oil or fat dissolved in the elution solvent (125 mg/mL) is introduced onto a glass column filled with a slurry of silica gel and elution solvent. A chromatographic glass column of 21- mm internal diameter and 450-mm length, and 25 g of silica gel - particle size 0.063-0.200 mm (70-230 mesh) - adjusted to a water content of 5% are used. The elution solvent is a mixture of light petroleum (b.p. 40-60°C) and diethyl ether 87:13 (v/v). Elution of nonpolar compounds, including mostly unoxidized TG, is carried out with 150 mL of the elution solvent and then the polar compound fraction is eluted with 150 mL of diethyl ether. A dropping funnel is used and the flow rate is adjusted to about 2.5 mL/min. Fractions are collected in round bottom flasks and solvents are removed with the aid of a rotary evaporator. Fractions can be dried to constant weight using a stream of nitrogen. The efficacy of the separation is assessed by thin-layer chromatography (TLC). Glass plates coated with silica gel, 0.25 mm layer, and a mixture of light petroleum, diethyl ether and acetic acid 70:30:2 (v/v/v) as developing solvent are used. After plate development and solvent evaporation, the plate is sprayed with a phosphomolybdic acid solution and heated at 120-130°C to visualise spots. As can be observed in Figure 1, an efficient separation is easily checked by confirming that unoxidized TG appear only in the first fraction and the spots of higher polarity can only be seen in the second fraction.
Collaborative tests conducted by the IUPAC demonstrated that the method for determination of polar compounds is exact and reproducible, showing coefficients of variation lower than 5% [3].
Similar good results have been obtained also in our laboratory after slight changes in the analytical procedure, such as replacement of light petroleum by hexane, small increases in the light petroleum:diethyl ether ratio to obtain a sharper separation, a final elution of the column with chloroform to improve recovery of the sample, or exposure to iodine vapours instead of use of phosphomolybdic acid and heat to visualise TLC spots [7].
The content of polar compounds is expressed in weight percentage of the starting oil and is calculated from the weights of the starting sample and nonpolar fraction as stated in the Standard Method or, alternatively, from the weights of nonpolar and polar fractions. Since polar compounds account for all the nonvolatile alteration compounds formed during frying, compounds originating from oxidation at high temperature (oxidized monomeric TG, dimeric and oligomeric TG) and compounds released from hydrolysis (fatty acids and diacylglycerols) are included (discussed here). In particular, dimers and oligomers are the most specific and major compounds in the polar fraction, and their quantitative importance increases with increasing degradation. Therefore, analysis of TG polymers, i.e. dimers plus higher oligomers, by high-performance size-exclusion chromatography (HPSEC) is also an excellent approach to determine alteration in used frying oils, and polymers levels have been found to correlate well with those of polar compounds separated by column chromatography [8].
3. Standardized Method Based on Minicolumns
For the sake of rapidity and reduced consumption of solvents and silica, the use of mini-columns was suggested [9], and later the IUPAC Commission decided to adopt the method [10]. The mini-column used is 10-mm internal diameter and 150-mm length, the oil sample is 0.5 g and the slurry consists on 5 g silica gel in about 10 mL of the elution solvent. Nonpolar and polar reactions are eluted with 60 mL of elution solvents and flow rate is adjusted to about 1.5 mL/min. The rest of the conditions are the same as those in the Standard IUPAC Method 2.507. Results from two collaborative studies, the first following the Standard Method 2.507 and the second one using the silica mini-columns, showed that, as expected, the higher the initial weight sample, 1 g for the Standard Method 2.507 and 0.5 g for the mini-column method, the lower was the standard deviation, as both methods are based on a gravimetric determination. However, the coefficient of variation for repeatability by using mini-columns was as low as 2% for a sample with about 30% polar compounds and 10% for the sample with the lowest content of polar compounds (3%).
4. Other Methods
Miniaturised methods, applicable to sample amounts below 100 mg and based on the use of solid-phase extraction (SPE) cartridges, have been proposed [11,12], but levels of accuracy and precision are expected to decrease for such low sample amounts, especially in samples with low levels of polar compounds.
However, when SPE is combined with HPSEC and addition of an internal standard, quantitative results significantly improve, as proposed in the analytical procedure developed in our laboratory [13]. It consisted of adding monostearin as internal standard and analysing the polar fraction by HPSEC. This is useful not only to determine the content of polar compounds but also to quantify different groups of compounds in the fraction. In this regard, combination of classical columns-HPSEC [7], micro-columns-HPSEC [10] and SPE-HPSEC [13] will be described in another web document.
Recently, we proposed a method based on application of HPSEC to the nonpolar fraction [14]. Starting from 30-50 mg oil sample with methyl oleate added as internal standard, the nonpolar fraction (containing unoxidized TG and methyl oleate) is obtained by SPE and quantitatively analysed by HPSEC. The polar fraction is determined by difference of weight. No significant differences were obtained as compared to results using the Standard IUPAC Method, and the repeatability was excellent.
5. Evaluation of Polar Compounds in Used Frying Oils
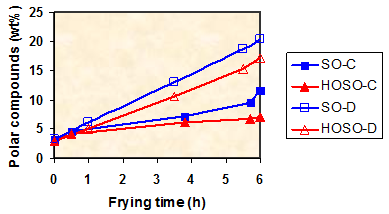
As an illustrative example of the application of polar compounds determination, Figure 2 shows the evolution of polar compounds in conventional sunflower oil and high-oleic sunflower oil during continuous and discontinuous frying [15]. Similar conditions of temperature (175°C), initial surface-to-volume ratio (0.3 cm-1) and total heating time (6 h) were applied. Differences between oils are expected from the different degree of unsaturation since the tocopherol contents were similar (620-650 mg tocopherols/kg oil). Remarkably high differences were found due to the frying process, attributable to the greater protection of the oil surface in continuous frying because of the constant presence of steam from water in the fried food.
Figure 2. Evolution of polar compounds during frying. SO-C, sunflower oil during continuous frying; HOSO-C, high-oleic sunflower oil during continuous frying; SO-D, sunflower oil during discontinuous frying; HOSO-D, high-oleic sunflower oil during discontinuous frying.
6. Conclusion
Determination of polar compounds by adsorption chromatography provides the most complete measure of the degradation level of used frying oils and fats, as it consists of the global quantification of the major alteration compounds formed. The method serves as the basis for limiting degradation of used frying oils and fats for human consumption in most national and international regulations, and it is extensively applied in both research and quality control laboratories.
Abbreviations: HPSEC = high-performance size-exclusion chromatography; SPE = solid-phase extraction; TG = triacylglycerols; TLC = thin-layer chromatography.
References
- Firestone, D. Regulation of frying fat and oil. In: Deep Frying: Chemistry, Nutrition and Practical Applications – 2nd Edition, pp. 373-385 (ed. M.D. Erickson, AOCS Press, Champaign) (2007).
- IUPAC (1987) Standard Method 2.507: Determination of polar compounds in frying fats. In: Standard Methods for the Analysis of Oils, Fats and Derivatives, 7th ed. (ed. International Union of Pure and Applied Chemistry, Blackwell, Oxford).
- Waltking, A.E. and Wessels, H. Chromatographic separation of polar and nonpolar components of frying fats. J. Assoc. Off. Anal. Chem., 64, 1329-1330 (1981).
- Guhr, G. and Waibel, J. Untersuchugen an fritierfetten; zusammenhänge zwischen dem gehalt an petroläter-unlöslichen oxidierten fett-säuren und dem gehalt an polaren substanzen bzw. dem gehalt an polymeren triglyceriden. Fette Seifen Anstrichm., 80, 106-113 (1978).
- German Society for Fat Science (DFG)-inheitsmethode C III 3b (84): Bestimmung der polaren Anteile in Fritierfetten.
- Bundesinstitut für gesundheitlichen Verbraucherschutz und Veterinärmedizin (BgVV): tierische und pflanzliche fette und öle, bestimmung des gehalts an polaren bestandteilen. amtliche sammlung von untersuchungsverfahren nach § 35 LMBG. Amtliche Methode L13.07.12-1 (Sept. 1997), =DIN/EN/ISO 8420 (Juli 1995).
- Dobarganes, M.C., Pérez-Camino, M.C. and Márquez-Ruiz, G. High performance size exclusión chromatography of polar compounds in heated and non-heated fats. Fat Sci. Technol., 20, 308-311 (1988).
- Marmesat, S., Rodrigues, E., Velasco, J., and Dobarganes, M.C. Quality of used frying fats and oils: comparison of rapid tests based on chemical and physical oil properties. Int. J. Food Sci. Technol., 42, 601-608 (2007).
- Dieffenbacher, A. and Martin, E. Determination of emulsifiers in foods: separation of polar and nonpolar lipids by chromatography on silica-gel microcolumns. Rev. Fr. Corps Gras, 34, 323-328 (1987).
- Dobarganes, M.C., Velasco, J. and Dieffenbacher, A. Determination of polar compounds, polymerized and oxidized triacylglycerols and diacylglycerols in oils and fats. Pure Appl. Chem., 72, 1563-1575 (2000).
- Sébédio, J.-L., Septier, C. and Grandgirard, A. Fractionation of commercial frying oil samples using Sep-Pak cartridges. J. Am. Oil Chem. Soc., 63, 1541–1543 (1986).
- Schulte, E. Micromethod for the gravimetric determination of polar components in frying fats with ready for use columns. Eur. J. Lipid Sci. Technol., 102, 574-579 (2000).
- Márquez, G., Jorge, N., Martín-Polvillo, M. and Dobarganes, M.C. Rapid, quantitative determination of polar compounds in fats and oils by solid-phase extraction and size-exclusion chromatography using monostearin as internal standard. J. Chromatogr. A, 749, 55-60 (1996).
- Marmesat, S., Velasco, J., Márquez-Ruiz, G. and Dobarganes, M.C. A rapid method for determination of polar compounds in used frying fats and oils. Grasas Aceites, 58, 179-183 (2007).
- Jorge, N., Márquez-Ruiz, G., Martín-Polvillo, M., Ruiz-Méndez, M.V. and Dobarganes, M.C. Influence of dimethylpolysiloxane addition to frying oils: performance of sunflower oil in discontinuous and continuous laboratory frying. Grasas Aceites, 47, 20-25 (1996).
In This Section
- Oil Refining
- Action of Natural Antioxidants During Frying
- Formation of New Compounds During Frying - General Observations
- Formation of cyclic fatty acids during frying
- Formation of Epoxy-, Keto- and Hydroxy-Fatty Acids
- Formation of Volatiles and Short-Chain Bound Compounds
- Formation of Dimers and Oligomers
- Oxysterol Formation Frying Oils
- Structural Analysis of the Cyclic Fatty Acids Formed during Frying
- Cyclic Fatty Acids: Isolation and Quantitative Analysis in Food and Biological Tissues
- Analysis of Used Frying Oils and Fats by High-Performance Size-Exclusion Chromatography
- Analysis of Trans Polyunsaturated Fatty Acids
- Determination of Polar Compounds in Used Frying Oils and Fats by Adsorption Chromatography
- Determination of Oxidized Monomeric, Dimeric and Oligomeric Triacylglycerols; Diacylglycerols and Free Fatty Acids
- Separation and Quantification of Oxidized Monomeric, Dimeric and Oligomeric Fatty Acids
- Analysis of Oxidized Fatty Acids
- Analysis of Oxidized Sterols in Frying Oils
- Metabolism and Physiological Effects of Cyclic Fatty Acids Formed from Linoleic and alpha-Linolenic Acids during Frying
- Metabolism and Physiological Effects of Cyclic Fatty Acids Formed from Linoleic and alpha-Linolenic Acids during Frying
- Metabolism of Trans Polyunsaturated Fatty Acids Formed during Frying
- Biological Effects of Frying Oils Mediated by the Activation of Peroxisome Proliferator-Activated Receptors (PPAR)