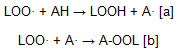Action of Natural Antioxidants During Frying
The Author: M. Carmen Dobarganes, Instituto de la Grasa (CSIC), Avda Padre Garcia Tejero 4, 41012 Seville, Spain. DOI: 10.21748/lipidlibrary.39208
1. Introduction
The action of phenolic antioxidants to delay lipid oxidation in fats and oils is well known although most of the information is related to their effects at room temperature during storage or at the moderate temperatures of accelerated tests. At room or moderate temperatures, autoxidation reactions taking place through chain reactions of free radicals are relatively slow; hydroperoxides are the major products formed and their concentration increases until advanced stages of oxidation. Under these conditions, the activity of primary antioxidants (AH) have been ascribed to the easy hydrogen transfer from their phenolic hydrogen to a peroxyl radical. Hydrogen transfer produces a radical, which might combine with another lipid peroxyl radical in a series of termination reactions yielding nonradical oxidation products as shown in equations [a] and [b].
However, oxidation at the high temperatures of food processes like frying is far more complex because both oxidative and thermal reactions are simultaneously involved. Chemical degradation of antioxidants seems to be the major pathway for antioxidant loss at high temperatures although volatilization and steam distillation due to both the high temperature and the large amount of steam water escaping from the food have also been postulated.
2. Tocopherols and Tocotrienols
The protection of tocopherols at the high temperatures of the frying process has been demonstrated by comparing the formation of new compounds in natural oils and tocopherol-stripped oils. For oils of different degrees of unsaturation, the degradation was significantly higher when the tocopherols were absent although their activity seemed to be more related to the type of natural tocopherols present in the oil than to the degree of oil unsaturation [1]. However, at low temperatures and at temperatures used in oxidative accelerated tests (100-120°C), the loss in tocopherols depended directly on the degree of oil unsaturation [2,3]. Thus, a mechanism dependent on temperature seems to be involved in their action [4].
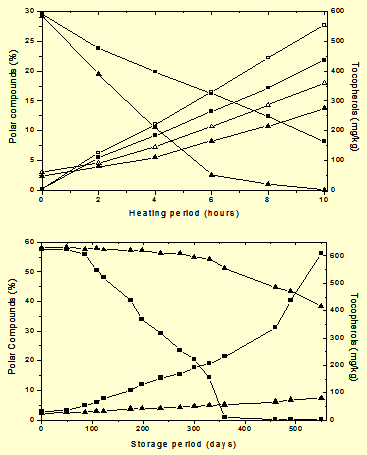
Figure 1 shows the different behavior of tocopherols at room and frying temperature in conventional high-linoleic and genetically modified high-oleic sunflower oils (Such oils were produced in particular sunflower lines for experimental purposes (see 21) and are not, currently, commercially available), i.e. oils with similar tocopherol composition but differing in the composition of triacylglycerols. Figure 1A shows the formation of polar compounds in the sunflower oils with and without their natural tocopherols heated at 180°C for 10 hours as well as the parallel loss of tocopherols when they were present in the samples [1]. Similar levels of tocopherols were present in both oils, more than 95% being α-tocopherol. The action of the natural antioxidants is clearly demonstrated by the higher levels of polar compounds of the stripped oils at any heating period. However, the effect of tocopherols in delaying the formation of polar compounds was not directly related to the degree of oil unsaturation. The most important aspect of the behaviour of tocopherols at frying temperature concerns their rapid exhaustion particularly in the less unsaturated oil. Unexpectedly, not only was the loss of tocopherols more rapid in the monounsaturated sunflower oil but also tocopherols were exhausted at lower oil degradation. These results have been reported in different studies either in model systems or for oils of different degrees of unsaturation and types of tocopherols [1,5-7]. The rapid loss of α-tocopherol suggests its substrate-independent degradation as the most important fact at frying temperature, probably with a secondary mode of action being the effective protection found on the formation of degradation compounds with respect to the tocopherol-stripped oils. In contrast, Figure 1B shows that, at room temperature, the formation of new compounds and the loss of tocopherols clearly depended on the degree of oil unsaturation, being more rapid in the more unsaturated sunflower oil.
Figure 1. Formation of polar compounds (increasing lines) and loss in natural tocopherols (decreasing lines) in high-linoleic (■) and high oleic (▲) sunflower oils. Oils heated at 180°C –hollow symbols correspond to tocopherol-stripped oils. Oils stored at room temperature.
From these results it is deduced that, on one hand, special attention should be paid to frying operations using monounsaturated oils, as they become unprotected at levels of polar compounds much lower than the limit established in official regulations for discarding fats for human consumption (25 wt% polar compounds). If the fried food has to be stored, oxidation at low temperature would be very rapid in the absence of antioxidants regardless of the degree of oil unsaturation [8]. On the other hand, these results indicate that accelerated tests at moderate temperatures or under the conditions of Rancimat tests may be useful to predict the relative shelf life of different oils and fats but they would not give an indication of their frying performance.
Concerning the type of tocopherols, the relative stability of α-, β-, γ- and δ-tocopherol at high temperatures has been studied in detail and there is agreement that α-tocopherol is less stable than δ-tocopherol, while β- and γ-tocopherols would degrade at intermediate rates. In this respect, new sunflower lines that contain γ-tocopherol as the major natural antioxidant instead of α-tocopherol, which is characteristic of standard sunflower oil, are of special interest [9].
The action of tocotrienols has been less studied. In general, the stability of α-tocotrienol was found to be similar to that of α-tocopherol both in palm olein and in purified oils to which both antioxidants had been added [10,11]. However, the order of stability of the different tocotrienol homologues was different to that found for their tocopherol counterparts, with γ-tocotrienol being the least stable [11].
Information on the fate of tocopherols at elevated temperatures is limited, which contrasts with the works on the chemistry of oxidation at room temperature or under the conditions of accelerated test without air limitation [12]. Although the mechanism of tocopherol loss at high temperatures is not fully understood, it has been reported that tocopherols have a very low volatility at frying temperature and hence, their rapid loss is due to their degradation. In this respect, the formation of α-tocopherolquinone, 4a,5-epoxy-α-tocopherolquinone, and 7,8-epoxy-α-tocopherolquinone has been demonstrated and their direct reaction with oxygen has been suggested [13].
3. Other Natural Antioxidants
There are three groups of natural antioxidants whose beneficial action in frying has been consistently reported. These are polyphenols in virgin olive oils, mainly comprised by hydroxytyrosol, tyrosol and their derivatives, and lignans [14] γ-oryzanol, i.e. sterol esters of ferulic acid, in rice bran oil [15]; and lignans, mainly sesamine, sesamolin and sesamol, in sesame oil [16].
The good performance of virgin olive oil in frying as compared to refined olive oil is mainly attributed to the presence of polyphenols. Interestingly, studies analyzing the evolution of tocopherols and polyphenols during frying reported a high rate of degradation for hydroxytyrosol and its derivatives, a similar or lower rate for α-tocopherol and a low degradation rate for tyrosol, its derivatives and lignans [17]. Therefore, the effective protection found is probably more related to the group of less active antioxidants present, i.e. lignans and tyrosol and their derivatives, which remain longer at frying temperatures.
There have been a limited number of reports on the good frying performance of pure sesame and rice bran oil in spite of their wide consumption in populous Asian countries. A high number of recent studies, however, report a significant increase in oil thermostability, as well as a higher retention of natural tocopherols, in the blends of different oils with either sesame or rice bran oil [18].
Native sesame oil contains predominantly sesamin and sesamolin and a small amount of sesamol. Neither sesamolin nor sesamin has been found to possess any appreciable antioxidant activity. However, sesamolin may be hydrolyzed during frying, due to the food moisture, giving sesamol and sesaminol. Both compounds are highly stable and have a synergistic effect with tocopherols, decreasing oil degradation [19]. As for rice bran oil phenolic compounds, information on the expected hydrolysis of γ-oryzanol during frying has not been found. On the contrary, it has been reported that the content of oryzanol did not change in practice under usual home frying conditions, regardless of its initial content in the oil [20].
In summary, given the complexity of the frying process attention should be paid to the two following points:
- The action of antioxidants at frying temperature cannot be deduced from their behaviour at low or moderate temperatures.
- There is a rapid loss in antioxidants in oils with high contents of monounsaturated fatty acids and their exhaustion may take place at degradation levels lower than those recommended for discarding the used frying oils. Thus, special attention for maintaining a minimum level of antioxidants in fried products to be stored is necessary.
References
- Barrera-Arellano, D., Ruiz-Méndez, M.V., Velasco, J., Márquez-Ruiz, G. and Dobarganes, M.C. Loss of tocopherols and formation of degradation compounds at frying temperatures in oils differing in unsaturation degree and natural antioxidant content. J. Sci. Food Agric., 82, 1696-1702 (2002).
- Martín Polvillo, M., Méndez, G. and Dobarganes, M.C. Oxidative stability of sunflower oils differing in unsaturation degree during long-term storage at room temperature. J. Am. Oil Chem. Soc. 81, 577-583 (2004) (DOI: 10.1007/s11746-006-0944-1).
- Márquez-Ruiz, G., Martín-Polvillo, M., Velasco, J. and Dobarganes, M.C. Formation of oxidation compounds in sunflower and olive oils under oxidative stability index conditions. Eur. J. Lipid Sci. Technol., 110, 465-471 (2008) (DOI: 10.1002/ejlt.200700246).
- Marinova, E.M. and Yanishlieva, N.V. Effect of temperature on the antioxidative action of inhibitors in lipid autoxidation. J. Sci. Food Agric., 60, 313-318 (1992).
- Barrera-Arellano, D., Méndez, M.V., Méndez-Ruiz, G. and Dobarganes, M.C. Loss of tocopherols and formation of degradation compounds in triacylglycerol model systems heated at high temperature. J. Sci. Food Agric. 79, 1923-1928 (1999).
- Verleyen, T., Kamal-Eldin, A., Dobarganes, M.C., Verhe, R., Dewettinck, K. and Huyghebaert, A. Modeling of α-tocopherol loss and oxidation products formed during thermoxidation in triolein and tripalmitin mixtures. Lipids, 36, 719-726 (2001).
- Verleyen, T., Kamal-Eldin, A., Mozutaityte, R., Verhe, R., Dewettinck, K., Huyghebaert, A. and de Greyt, W. Oxidation at elevated temperatures: competition between α-tocopherol and unsaturated triacylglycerols. Eur. J. Lipid Sci. Technol., 104, 228-233 (2002).
- Márquez-Ruiz, G., Martín-Polvillo, M., Jorge, N., Ruiz Méndez, M.V. and Dobarganes, M.C. Influence of used frying oil quality and natural tocopherol content on oxidative stability of fried potatoes. J. Am. Oil Chem. Soc., 76, 421-425 (1999).
- Marmesat, S., Velasco, L., Ruiz-Méndez, M.V., Fernández-Martínez,, J.M. and Dobarganes, M.C. Thermostability of genetically modified sunflower oils differing in fatty acid and tocopherol compositions. Eur. J. Lipid Sci. Technol. 110, 776-782 (2008) (DOI: 10.1002/ejlt.200800040).
- Romero, N., Robert, P., Masson, L., Ortiz, J., González, K., Tapia, K. and Dobarganes, M.C. Effect of α-tocopherol, α-tocotrienol and Rosa mosqueta shell extract on the performance of antioxidant-stripped canola oil (Brassica sp.) at high temperature. Food Chem., 104, 383-389 (2007) (DOI: 10.1016/j.foodchem.2006.11.052).
- Rossi, M., Alamprese, C. and Ratti, S. Tocopherols and tocotrienols as free radical-scavengers in refined vegetable oils and their stability during deep-fat frying. Food Chem., 102, 812-817 (2007) (DOI: 10.1016/j.foodchem.2006.06.016).
- Kamal-Eldin, A. and Appleqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids, 31, 671-699 (1996).
- Verleyen, T., Verhe, R., Huyghebaert, A., Dewettinck, K. and de Greyt, W. Identification of α-tocopherol oxidation products in triolein at elevated temperatures. J. Agric. Food Chem., 49, 1508-1511 (2001).
- Cicerale, S., Conlan, X.A., Sinclair, A.J. and Keast, R.S.J. Chemistry and health of olive oil phenolics. Crit. Rev. Food Sci. Nutr., 49, 218-236 (2009) (DOI: 10.1080/10408390701856223).
- Lerma-García, M.J., Herrero-Martínez, J.M., Simó-Alfonso, E.F., Mendonça, C.R.B. and Ramis-Ramos, G. Composition, industrial processing and applications of rice bran γ-oryzanol. Food Chem., 115, 389-404 (2009) (DOI: 10.1016/j.foodchem.2009.01.063).
- Namiki, M. Nutraceutical functions of sesame: a review. Crit. Rev. Food Sci. Technol., 47, 651-673 (2007) (DOI: 10.1080/10408390600919114).
- Brenes, M., García, A., Dobarganes, M.C., Velasco, J. and Romero, C. Influence of thermal treatments simulating cooking processes on the polyphenol content of virgin olive oil. J. Agric. Food Chem., 50, 5962-5967(2002).
- Chung, J., Lee, Y. and Choe, E. Effects of sesame oil addition to soybean oil during frying on the lipid oxidative stability and antioxidants contents of the fried products during storage in the dark. J. Food Sci., 71, C222-C226 (2006) (DOI: 10.1111/j.1365-2621.2006.tb15621.x).
- Yoshida, H. and Takagi, S. Antioxidative effects of sesamol and tocopherols at various concentrations in oils during microwave heating. J. Sci. Food Agric. 79, 220-226 (1999).
- Krishna, A.G.G., Khatoon, S. and Babylatha, R. Frying performance of processed rice bran oils. J. Food Lipids, 12, 1-11 (2005) (DOI: 10.1111/j.1745-4522.2005.00001.x).
- J.J.Salas et al.(2014) Biochemistry of high stearic sunflower, a new source of saturated fats. Prog. Lipid Res. 55,30-42.
In This Section
- Oil Refining
- Action of Natural Antioxidants During Frying
- Formation of New Compounds During Frying - General Observations
- Formation of cyclic fatty acids during frying
- Formation of Epoxy-, Keto- and Hydroxy-Fatty Acids
- Formation of Volatiles and Short-Chain Bound Compounds
- Formation of Dimers and Oligomers
- Oxysterol Formation Frying Oils
- Structural Analysis of the Cyclic Fatty Acids Formed during Frying
- Cyclic Fatty Acids: Isolation and Quantitative Analysis in Food and Biological Tissues
- Analysis of Used Frying Oils and Fats by High-Performance Size-Exclusion Chromatography
- Analysis of Trans Polyunsaturated Fatty Acids
- Determination of Polar Compounds in Used Frying Oils and Fats by Adsorption Chromatography
- Determination of Oxidized Monomeric, Dimeric and Oligomeric Triacylglycerols; Diacylglycerols and Free Fatty Acids
- Separation and Quantification of Oxidized Monomeric, Dimeric and Oligomeric Fatty Acids
- Analysis of Oxidized Fatty Acids
- Analysis of Oxidized Sterols in Frying Oils
- Metabolism and Physiological Effects of Cyclic Fatty Acids Formed from Linoleic and alpha-Linolenic Acids during Frying
- Metabolism and Physiological Effects of Cyclic Fatty Acids Formed from Linoleic and alpha-Linolenic Acids during Frying
- Metabolism of Trans Polyunsaturated Fatty Acids Formed during Frying
- Biological Effects of Frying Oils Mediated by the Activation of Peroxisome Proliferator-Activated Receptors (PPAR)