Determination of Oxidized Monomeric, Dimeric and Oligomeric Triacylglycerols; Diacylglycerols and Free Fatty Acids
The Author: Gloria Márquez-Ruiz, Instituto de Ciencia y Tecnología de Alimentos y Nutrición (ICTAN-CSIC), José Antonio Novais, 10, 28040 Madrid, Spain, DOI: 10.21748/lipidlibrary.39199
1. Introduction
Determination of polar compounds by adsorption chromatography and further analysis of the polar fraction by high-performance size-exclusion chromatography (HPSEC) allows quantification of the most abundant groups of new compounds formed during frying. The new compounds quantified are those resulting from oxidation and thermal alterations taking place in the unsaturated fatty acids (oxidized monomeric, dimeric and oligomeric triacylglycerols (TG)) and those released through hydrolytic reactions (free fatty acids, monoacylglycerols and diacylglycerols). In this article, the basis of this combined analytical procedure and variations proposed for adsorption chromatographic separation (silica columns or solid-phase extraction) are described. Also, advantages of the analytical procedure and their main applications in used frying oils and fats are discussed.
2. Combination of Classical Silica Columns and HPSEC
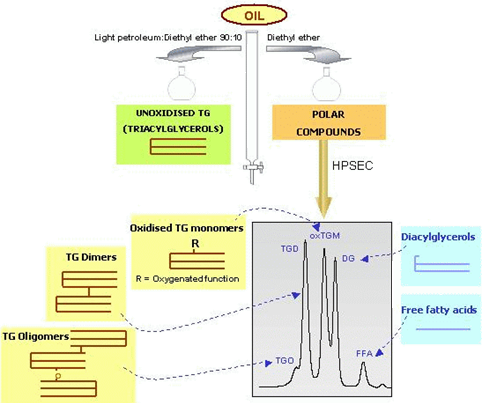
The analytical procedure is based on two IUPAC methods, i.e. determination of polar compounds [1] and determination of polymers [2], and was developed in our lab with the aim of providing quantitative data on groups of compounds characteristic of the different types of degradation in used frying oils and fats [3]. The procedure is described briefly below and is shown schematically in Figure 1. A chromatographic glass column of 21 mm internal diameter and 450 mm length is used. Starting from 1 g oil, nonpolar and polar fractions are eluted with 150 mL of a mixture petroleum ether-diethyl ether (90:10, v/v) and 150 mL diethyl ether, respectively. The efficacy of the separation is assessed by thin-layer chromatography (TLC). After gravimetric determination of polar compounds, the polar fraction is further analyzed by HPSEC, using 100 and 500 Å pore size columns with a polystyrene-divinylbenzene highly cross-linked macroporous packing (particle size: 5 mm) connected in series; mobile phase: tetrahydrofuran (flow rate: 1 mL/min); detector: refractive index.
Figure 1. Methodology based on combination of silica columns and HPSEC.
As result of the combined separation criteria used, firstly based on polarity and secondly on molecular size or weight, five groups of compounds are neatly separated and quantified: TG oligomers (TGO), TG dimers (TGD), oxidised TG monomers (oxTGM), diacylglycerols (DG), and free fatty acids (FFA), in this order of elution. TG oligomers are polymers constituted by three or more TG molecules. The peak containing FFA includes also polar unsaponifiable matter. The groups of compounds quantified can be differentiated between thermally oxidised compounds (oxTGM, TGD and TGO) and hydrolytic products (DG and FFA). Contribution of both types of products to the amount of polar compounds helps determine the real nutritional significance of the 25% polar compounds limitation normally established for frying oils, since thermally oxidized products are those associated with negative physiological effects while hydrolytic compounds are products naturally released from lipolysis in the gut before absorption. Concentrations are calculated from peak areas and gravimetric determination of the polar fraction.
In Figure 1, an example of a simplified structure is shown for each group of compounds. In reality, each peak includes a high number of different structures. For example, the original diversity in fatty acid and TG composition plus the nature, location and number of oxygenated functions formed give rise to a multitude of compounds included in the oxTGM peak. Further, TGD and TGO may possess C-C or C-O-C linkages in different fatty acyl chain locations, and the potential number of possible combinations of the intermediate oxTGM and TG structures increases exponentially with the degree of polymerization (see our web page on Formation of Dimers and Oligomers).
3. Combination of Silica Minicolumns and HPSEC
The methodology later standardised by the IUPAC [4] is very similar to that described above and differs mainly in the use of smaller columns (minicolumns), which permits starting from lower amount of sample and requires lower elution volumes (see our web page on Determination of Polar Compounds by Adsorption Chromatography). The chromatographic glass column is 10 mm internal diameter and 150 mm length, the sample amount is 0.5 g, and elution volumes are 60 mL of a mixture petroleum ether-diethyl ether (90:10, v/v) and 50 mL of diethyl ether, for the nonpolar and polar fractions, respectively. Two collaborative studies showed excellent repeatability and reproducibility of results. Even though higher standard deviations for determination of polar compounds were obtained through the minicolumn method than for the Standard Method 2.507, as expected from the higher initial weight sample in the latter case, repeatability and reproducibility for oxTGM, DG and TG polymers were high and of the same order.
4. Combination of Solid Phase Extraction and HPSEC
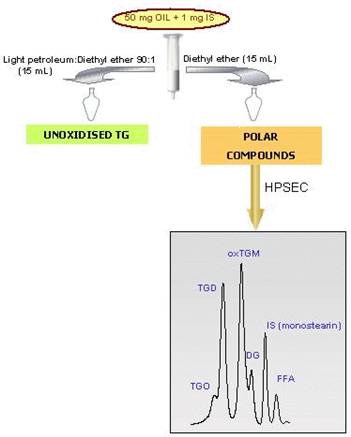
Silica column separation can be replaced by solid phase extraction (SPE) thus requiring even lower amounts of sample, solvents and analysis time. This alternative possibility was proposed for 50 mg-samples, based on the use of SPE silica gel cartridges for the separation of nonpolar and polar fractions, and the addition of an internal standard for quantification purposes [5]. Briefly, 2 mL of the sample solution, containing 50 mg of oil and 1 mg of monostearin (internal standard), is placed on the silica cartridge for SPE (Fig. 2). Monostearin was used as internal standard because MG are normally in negligible or undetectable amounts in fats and oils. The nonpolar fraction is eluted with 15 mL of petroleum ether-diethyl ether (90:10, v/v). A second fraction containing polar compounds and the internal standard is eluted with 15 mL of diethyl ether. The rest of conditions are the same than those described previously.
Figure 2. Methodology based on combination of solid phase extraction and HPSEC.
Precision, accuracy and recovery data were determined. Samples containing levels of polar compounds ranging from 3.7 to 24.3% were analysed by both this procedure and the Standard Method 2.507, and no significant differences were found. However, the lower the polar compound concentration, the lower the standard deviations found for the internal standard method. Therefore, this modified procedure is especially useful for samples with low levels of degradation.
5. Advantages of Combination of Adsorption Chromatography (Classical Columns, Minicolumns or SPE) and HPSEC
- By virtue of the previous separation of the most abundant fraction of the sample, i.e. the unoxidized TG, application of HPSEC to the concentrated fraction of polar compounds allows quantification of different groups of oxidized and hydrolytic compounds. For example, when HPSEC is applied directly to a used frying oil, only TG dimers and oligomers can be determined, since abundant unoxidized TG co-elute with oxTGM and overlap with DG. This can be easily observed by comparing chromatograms in Figure 1 and Figure 2 B in the webpage dealing with Analysis of Used Frying Oils and Fats by High-Performance Size-Exclusion Chromatography, corresponding to the polar fraction and the whole used frying oil, respectively.
- The analytical procedure allows determination of oxTGM, i.e. TG containing one or more oxygenated function in at least one of the fatty acyl chains, which come from the decomposition of primary oxidation compounds (hydroperoxides), unstable at frying temperatures. The main functional groups present in oxTGM of frying oils are epoxy, keto and hydroxy (see our web page on Formation of Epoxy-, Keto- and Hydroxy-Fatty Acids). The importance of oxTGM resides in both the high amounts formed during frying and their association with negative physiological effects. The amount of oxTGM in used frying fats and oils around the limit of rejection (25% polar compounds) has been shown to be very high, ranging from 5.9% to 9.4% on oil [6].
- The analytical procedure allows determination of DG, which are relevant in that they are useful markers of hydrolytic degradation.
-
Finally, a substantial increase in sensitivity is achieved in quantification of polymerisation compounds due to the effect of concentration, overcoming the limitation of the IUPAC Standard Method 2.508 to a minimum content of 3% for analyses of total samples by HPSEC [2].
6. Applications
In recent years, a plethora of studies on frying fats and oils based on application of this methodology has been published. Recent reviews contain all the relevant references [7-9]. Results obtained have contributed to improved knowledge of important issues of the frying process, such as the frying performance of different oils, composition of oils absorbed by the fried food and lipid interchange between frying oil and food, effect of antioxidants during frying, and the action of the main variables involved in the continuous and discontinuous frying processes.
Besides applications in used frying fats and oils, the analytical methodology serves to evaluate frying oils before use [10]. Even if the fraction of polar compounds in refined fats and oils is low, as TG normally constitute more than 95% of the sample, four peaks of significance can be well resolved: TGD, formed during the deodorization step but almost absent from crude oils; oxTGM and DG, quality markers of crude oils since they remain after refining; and FFA, decreasing with respect to the crude oils due to the neutralisation step (see our web page on Oil Refining). In contrast with the quality specifications established for frying oils before use, which only serve for checking if the oils are well refined, quantification of TGD, oxTGM and DG providesinformation on the polymerisation, oxidation and hydrolysis levels of refined oils, which could affect their performance during frying.
Table 1 shows data obtained in used frying oils of different alteration levels and three unused oils, selected to illustrate the profiles of compounds typically obtained [3,6,11,12]. Differences are observed in unused oils, i.e. most polar compounds are oxTGM and TGD in refined sunflower and soybean oils, while DG are the most abundant in olive oil. Among used frying oils collected by Food Inspection Services, samples D (sunflower oil) and E (olive oil), with the same level of polar compounds (27.6%), showed a different pattern of compound contribution, with greater polymerisation in the case of sunflower oil. Overall, it has been found that polymers (TGO+TGD) are by far the most abundant compounds in used frying oil samples [13].
| Table 1. Polar compounds content and distribution in unused oils and used frying oils | ||||||||||||
| Sample | Polar compounds (wt%) | TGO (wt%) | TGD (wt%) | OxTGM (wt%) | DG (wt%) | FFA (wt%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unused olive oil | 2.8 | nd | nd | 0.6 | 1.7 | 0.5 | ||||||
| Unused sunflower oil | 3.4 | nd | 0.9 | 1.6 | 0.7 | 0.2 | ||||||
| Unused soybean oil | 5.1 | nd | 0.5 | 2.9 | 1.3 | 0.4 | ||||||
| Used frying oil A | 14.5 | 1.1 | 5.4 | 5.6 | 2.0 | 0.4 | ||||||
| Used frying oil B | 17.4 | 2.1 | 7.7 | 5.9 | 1.2 | 0.5 | ||||||
| Used frying oil C | 25.5 | 4.7 | 9.0 | 8.6 | 2.6 | 0.6 | ||||||
| Used frying oil D | 27.6 | 6.5 | 10.9 | 7.2 | 2.3 | 0.6 | ||||||
| Used frying oil E | 27.6 | 3.7 | 7.3 | 9.4 | 6.2 | 1. | ||||||
| Used frying oil F | 30.8 | 5.9 | 9.8 | 11.5 | 3.2 | 0.4 | ||||||
7. Conclusion
At present, determinations of the main groups of compounds, namely oxidized, polymerized and hydrolytic compounds, by combinations of adsorption and size-exclusion chromatographies, constitute the best analytical tools for quantification of the most abundant compounds formed during frying and allow evaluation of the relative importance of the main pathways of degradation. The methods are also useful for the analysis of frying oils before use.
References
- IUPAC (1987) Standard Method 2.507: Determination of polar compounds in frying fats. In: Standard Methods for the Analysis of Oils, Fats and Derivatives, 7th ed. (ed. International Union of Pure and Applied Chemistry, Blackwell, Oxford).
- IUPAC (1992) Standard Method 2.508: Determination of polymerized triglycerides in oils and fats by high performance liquid chromatography. In: Standard Methods for the Analysis of Oils, Fats and Derivatives, 7th ed. (ed. International Union of Pure and Applied Chemistry, Blackwell, Oxford).
- Dobarganes, M.C., Pérez-Camino, M.C. and Márquez-Ruiz, G. High performance size exclusion chromatography of polar compounds in heated and non-heated fats. Fat Sci. Technol., 20, 308-311 (1988).
- Dobarganes, M.C., Velasco, J. and Dieffenbacher, A. Determination of polar compounds, polymerized and oxidized triacylglycerols and diacylglycerols in oils and fats. Pure Appl. Chem., 72, 1563-1575 (2000).
- Márquez-Ruiz, G., Jorge, N., Martin-Polvillo, M. and Dobarganes, M.C. Rapid, quantitative determination of polar compounds in fats and oils by solid-phase extraction and size-exclusion chromatography using monostearin as internal standard. J. Chromatogr. A, 749, 55-60 (1996).
- Márquez-Ruiz, G., Tasioula-Margari, M. and Dobarganes, M.C. Quantitation and distribution of altered fatty acids in frying fats. J. Am. Oil Chem. Soc., 72, 1171-1176 (1995).
- Dobarganes, M.C. and Márquez-Ruiz, G. Formation and analysis of oxidized monomeric, dimeric and higher oligomeric triglycerides. In: Deep Frying: Chemistry, Nutrition and Practical Applications – 2nd Edition, pp. 87-110 (ed. M.D. Erickson, AOCS Press, Champaign) (2007).
- Márquez-Ruiz, G. and Dobarganes, M.C. High-performance size-exclusion chromatography for lipid analysis in organic media. In: Lipid Analysis and Lipidomics: New Techniques and Applications, pp. 205-238 (eds. M.M. Mossoba, J.K.G. Kramer, J.T. Brenna and R.E. McDonald, AOCS Press, Champaign, USA) (2006).
- Márquez-Ruiz, G. and Dobarganes, M.C. Analysis of nonvolatile lipid oxidation compounds by high-performance size-exclusion chromatography. In: Analysis of Lipid Oxidation, pp. 40-69 (eds. A. Kamal-Eldin and J. Pokorný, AOCS Press, Champaign, USA) (2005).
- Ruiz-Méndez, M.V., Márquez-Ruiz, G. and Dobarganes, M.C. Relationships between quality of crude and refined edible oils based on quantitation of minor glyceridic compounds. Food Chem., 60, 549-554 (1997).
- Ruiz-Méndez, M.V., Marmesat, S., Liotta, A. and Dobarganes, M.C. Analysis of used frying fats for the production of biodiesel. Grasas Aceites, 59, 45-50 (2008).
- Rodrigues-Machado, E., Marmesat, S., Abrantes, S. and Dobarganes, M.C. Uncontrolled variables in frying studies: differences in repeatability between thermoxidation and frying experiments. Grasas Aceites, 58, 283-288 (2007).
- Marmesat, S., Rodrigues-Machado, E., Velasco, J. and Dobarganes, M.C. Quality of used frying fats and oils: comparison of rapid tests based on chemical and physical oil properties. Int. J. Food Sci. Technol., 42, 601-608 (2007).
In This Section
- Oil Refining
- Action of Natural Antioxidants During Frying
- Formation of New Compounds During Frying - General Observations
- Formation of cyclic fatty acids during frying
- Formation of Epoxy-, Keto- and Hydroxy-Fatty Acids
- Formation of Volatiles and Short-Chain Bound Compounds
- Formation of Dimers and Oligomers
- Oxysterol Formation Frying Oils
- Structural Analysis of the Cyclic Fatty Acids Formed during Frying
- Cyclic Fatty Acids: Isolation and Quantitative Analysis in Food and Biological Tissues
- Analysis of Used Frying Oils and Fats by High-Performance Size-Exclusion Chromatography
- Analysis of Trans Polyunsaturated Fatty Acids
- Determination of Polar Compounds in Used Frying Oils and Fats by Adsorption Chromatography
- Determination of Oxidized Monomeric, Dimeric and Oligomeric Triacylglycerols; Diacylglycerols and Free Fatty Acids
- Separation and Quantification of Oxidized Monomeric, Dimeric and Oligomeric Fatty Acids
- Analysis of Oxidized Fatty Acids
- Analysis of Oxidized Sterols in Frying Oils
- Metabolism and Physiological Effects of Cyclic Fatty Acids Formed from Linoleic and alpha-Linolenic Acids during Frying
- Metabolism and Physiological Effects of Cyclic Fatty Acids Formed from Linoleic and alpha-Linolenic Acids during Frying
- Metabolism of Trans Polyunsaturated Fatty Acids Formed during Frying
- Biological Effects of Frying Oils Mediated by the Activation of Peroxisome Proliferator-Activated Receptors (PPAR)