Optimization of Bleaching Process
The Authors: David D. Brooks, Roberto Berbesi and Allan S. Hodgson
Introduction
The bleaching of edible oils and fats is a part of the refining process of crude oils and fats, which removes contaminants that adversely impact the appearance and performance of these triglyceride (triacylglycerol)-based materials. Typically, edible oils and fats, ranging from soybean and palm oils to edible lard and beef tallow, are extracted together with impurities in various quantities. Many of these impurities have to be removed from the oil to achieve the high quality oil standards necessary for edible applications. Preceded generally by degumming and refining (neutralization) processes, bleaching is required to remove specific detrimental contaminants that are not effectively removed by these processes before the oil progresses through deodorization.
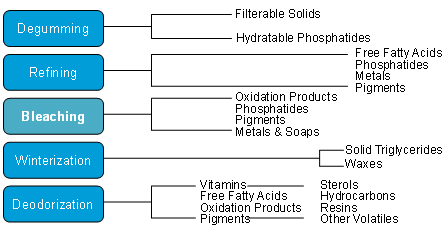
Originally described as a process of mixing oil and clay adsorbent to remove color, the bleaching operation effectively removes some of the color, reduces the contents of chlorophyll, residual soap and gums, trace metals, oxidation products, and indirectly impacts on deodorized oil color. While the bleaching process appears to be a simple mixing of adsorbent and oil followed by filtration, the chemical and physical reactions occurring are complex and greatly reliant on process variables (i.e. moisture levels, temperature, contact time, and vacuum), oil quality entering the bleacher, the amount and characteristics of the adsorbent and the type of equipment employed. The success or efficiency of the bleaching operation is interdependent on the effectiveness of upstream processes where contaminants that have the potential to interfere with the bleaching mechanisms should be removed (Fig. 1) [1]. Some consider bleaching the “safety net” of the refining process in that it is the last operation in the oil refining process before going to deodorization. As the focus of this paper would suggest, optimization of the bleaching process is considerably important to both (1) achieving high quality refined oil products and (2) the economic viability of the oil purification process.
Figure 1. Processing efficiency - process-dependent removal of contaminants.
Bleaching Clay – Characteristics
The adsorptive capacity of sorbent minerals depends on their mineralogical structure and adsorptive properties, such as surface area, particle size distribution, porosity, and surface acidity. Bleaching clays or earths are generally composed of one or more of three types of clay minerals: calcium montmorillonite, attapulgite, and sepiolite. Microscopic views of two of these clay-based minerals are depicted in Figure 2. One is calcium montmorillonite (commonly referred to as bentonite) and the other is a naturally occurring blend of attapulgite and montmorillonite from the Georgia region in the USA.
Figure 2. Bleaching clay base minerals.
Bentonite minerals have limited sorptive properties in the natural state and require chemical treatment by acids to create the surface area and porosity needed for bleaching vegetable oils. Bleaching clays of this nature are commonly referred to as “acid” or “acid-activated” clays.
Attapulgite and sepiolite minerals have a naturally high affinity for adsorbing oil contaminants without any acid treatment. These natural clays can be acidified with mineral acids as well as used in conjunction with chelating acids such as citric or phosphoric acids to improve bleaching activity with respect to chlorophyll.
Bleaching Clay – Activation
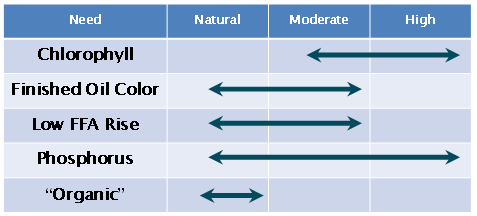
Bleaching clays are activated to varying degrees via interaction with acids ranging from completely natural clays, as explained above, to highly acid-treated clays (Fig. 3). The benefits to having increased adsorbent activity and/or impurity removal (i.e. bleaching efficiency) improve as the clay acidity of a base mineral is increased (pH lowers with limit approaching pH 2). However, employing acid-activated clays can have detrimental side effects in the oil; these include increased levels of free fatty acids [2,3] and the formation of undesirable 3-monochloropropane-1,2-diol esters (MCPD) [4-6]. Natural clays are often preferred by refineries that are sensitive to these contaminants.
Figure 3. Influence of bleaching earth activation levels. Typical activation levels for quality variables.
Furthermore, natural clays are the only bleaching clay option that meets the “Organic” oil certification because their manufacture does not involve any restricted chemical agent. In some situations, “organic” approved natural acids such as citric acid can further enhance the bleaching effectiveness of natural clays and still meet “organic” labeling requirements for chlorophyll-rich oils. This acid can be added to the oil being bleached, or acid-treated clay can be prepared in advance.
Bleaching Mechanisms
During the bleaching process adsorption occurs via many different mechanisms involving various physical and chemical interactions [1]; most of them improve the quality of the oil, but some of them may reduce it (Fig. 3). These mechanisms include the following:
Adsorption ─ mechanism by which the sorbent binds a contaminant. This can occur in three different ways:
- Physically through surface attraction involving van der Waals' forces
- Chemically by “chemisorption” by electrochemical bonding to the surface of the clay
- By molecular sieves which trap contaminants under pressure inside the pores of the clay during filtration
Absorption ─ mechanism by which the intra-granular pores are filled with some fluid—mainly oil — and in turn whatever contaminants came along with it. Oil retention is reported in two ways: as weight loss by Soxhlet extraction (with hexane being used as solvent), and as total organic matter determined by ashing.
Total oil retention depends on a number of variables including clay dosage levels, clay characteristics (e.g. particle size distribution and mineral type), permeability of the filter bed, incoming feedstock quality, cleanliness of the filter screen and the conditions used to purge the filter before disposal of the “spent” filter cake. Excessive oil retention increases the cost of running the process; oil loss through spent earth can typically range up to 35 weight percent solvent extractable and 50 weight percent for total organic matter of the earth used.
Filtration ─ mechanism of trapping or physically removing suspended contaminants: the physical act of filtering out the suspended clay that simultaneously removes the minor contaminants adsorbed to the clay particles. Filters used in the bleaching process include (1) processing filters (horizontal and vertical leaf filters) and (2) polishing filters (bag, cartridge, paper).
Catalysis ─ mechanism by which contaminants are degraded by interaction with the surface of the clay. For example, peroxides are effectively reduced (polymerized and/or decomposed into volatile oxidation by-products) by clay/oil interaction. With excessive heat and oxidation, pigments can form color compounds that are difficult to remove or said to be “fixed.” In the event of color fixation, red color is more difficult to remove by bleaching clays alone and resistant to thermal degradation leading to higher red color after deodorization.
Process Options
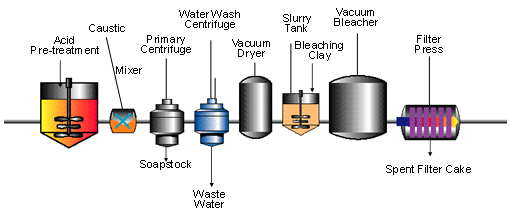
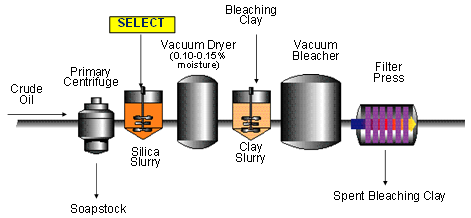
The bleaching process configurations have advanced from the slow, less efficient batch process to the highly efficient countercurrent process options including true countercurrent and lead-lag or double pass bleaching systems currently offered and discussed in the literature (see Figs. 4-8). Batch bleaching systems continue to be the process of preference for those who process many different products per day in small quantities. Countercurrent bleaching systems [7] find more favor in modern facilities because they maximize bleaching efficiency by utilizing the spent clay to exhaustion, which results in substantial savings (up to 40% reported) with 20-30% reported clay reduction [8].
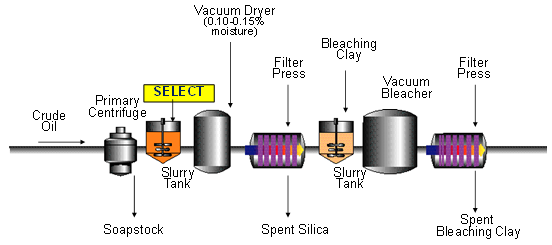
Figure 4. Continuous bleaching [1].
Figure 5. Silicate/clay bleaching - single filtration bleaching [1].
Figure 6. Silicate/clay bleaching - dual filtration bleaching [1].
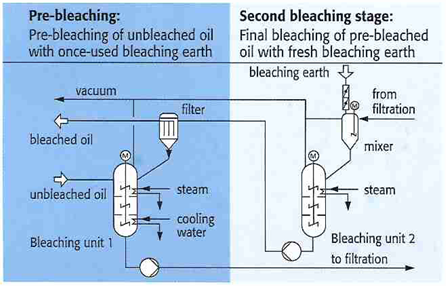
Figure 7. Countercurrent bleaching system [7].
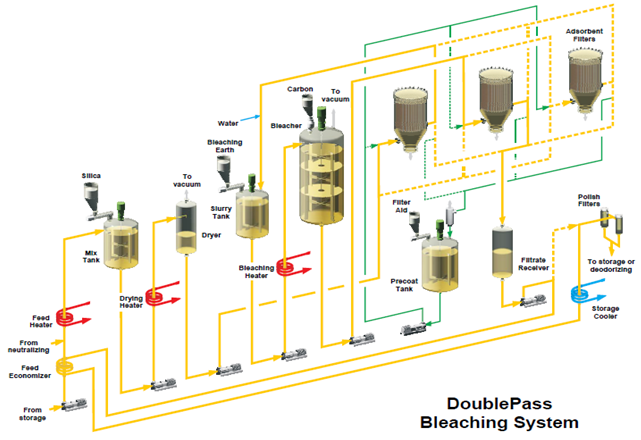
Figure 8. Double-pass bleaching system [8].
Improved Oil Quality
Bleaching is the physical and chemical interaction of an oil or fat with bleaching earth to improve its quality. One definition of quality is “to reach a level of excellence.” However, with respect to oil specifications, it is quite variable and depends on the product and market we are dealing with. For example, for deep-frying oils, it is very important that phosphorus (P) be as low as possible and a residual content of <0.5 ppm is desired, but for salad oil, a level of <2 ppm may be specified.
Various processing conditions can affect one or more of the oil characteristics that have been listed in quality specifications. By balancing these conditions one against the other, you will be one step closer to a more efficient bleaching process.
To arrive at an optimal bleaching process, you must first establish what the primary target specifications will be and make sure that you are getting the appropriate contaminants removed by the overall refining process (see Fig. 1). Good refined oil must be [1] :
- Low in phosphorus (good if <15 ppm, very good if <10 ppm and excellent if <5 ppm)
- Low in free (unesterified) fatty acids (FFA <0.1 % unless some adaptation of physical refining is being employed)
- Low in soaps (<50 ppm unless silica/silicate refining is used)
Once you have this under control you can begin the bleaching stage where:
- Soaps are completely removed
- Phosphorus content is reduced to below 2 ppm
- Iron content is reduced to below 0.2 ppm
- Chlorophyll content is reduced to below 0.05 ppm
- Peroxide Value is reduced to below 0.5 mEq/kg
All values cited herein serve as industry guidelines to meet product specifications. Each refinery is unique, having any assortment of equipment and production lines set up to meet customer driven specifications.
Understanding Interactions
There are numerous interactions between the process variables that influence the removal of oil contaminants and the efficiency of the bleaching process. A single change of a given operational parameter can cause many changes in the oil properties at the same time — some good and some bad. The following sections will show the effect of the most common processing variables on the most common quality parameters.
Controlling the Process
Clay dosing in bleaching operations is typically controlled by monitoring bleached oil red color and/or chlorophyll; they are two of the easiest parameters to measure.
Color
The color of an oil or fat depends on the quality of the source material from which it is extracted. Hues can range from orange (palm oil) to browns (rendered fats). Tintometers®, both manual and automatic, ascribe comparative numerical values relative to the hue and shade of a given sample to those of a series of tinted glasses of specific colors and intensities (dependent on cell path length); the most universally applied system is the Lovibond Tintometer® which assigns a red/yellow color (as in soy, corn, rapeseed) or a red-yellow-blue neutral color (as in tallows and soaps). In oils where yellow hues have a significant contribution to overall color along with the red component, refineries have employed the color index; (Red + Yellow/10) / 2 = Color index).
Chlorophyll content
The “chlorophyll” concentration of bleached oils can be determined through photometric techniques employed if need be in combination with high-performance liquid chromatography (HPLC). The most common and economical of these techniques is the Official AOCS method (Cc13e-92) [9] based on spectrophotometric absorbance readings at 630, 670 and 710 nm. This method can be performed on a spectrophotometer as well as on a programmed automated Tintometer® [10].
Color vs. Chlorophyll
Monitoring bleached oil red color is not the best way to control and predict bleaching performance; the exception being when your process and feedstock are completely stable (consistent). Monitoring the chlorophyll content after bleaching is currently a more common practice for optimizing the bleaching process than monitoring the red color of bleached oils.
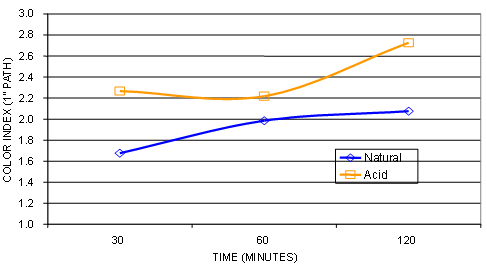
There is enough evidence proving that, for a given type of oil, a higher red color of the bleached oil does not necessarily result in a higher red color of the deodorized oil; this depends on the processing conditions used (see Fig. 9) and occurs especially with corn and palm oils. Additionally, bleached oil red color does not correlate with chlorophyll content. Refineries that only monitor the red color on occasion fail to meet final oil red and yellow color due to the masking effect of green color from chlorophyll. Oil extracted from green beans can have excessive amounts of chlorophyll and fail to meet final oil color specifications on the red and yellows especially when oil with a higher than normal level of chlorophyll passes through the deodorizer.

Figure 9. Bleaching temperature vs. color comparison of refined bleached (RB) (upper) vs. refined bleached & deodorized (RBD) (lower) corn oils.
Temperature
Oil bleaching temperatures typically range from 90–125°C (195–257°F). Temperature affects oil viscosity and adsorption kinetics. Oil viscosity decreases with increasing temperature resulting in better dispersion of bleaching earth particles, improved clay/oil interactions, and less resistance to flow. The ability to maintain a particle in slurry suspension is inversely related to the viscosity of the oil, implying that clays with coarser particle size distributions (PSD) will require a more powerful agitation to stay in suspension and that clays with finer PSDs may more rapidly disperse into the oil at higher temperatures. A higher temperature may give you benefits on chlorophyll removal, color, and filtration rates, however, depending on oil type and oil quality, may in turn worsen the color of the deodorized oil, and its oxidative stability.
Contact time
The contact time between the oil and the bleaching earth refers to the total time that the bleaching clay is in contact with the oil going from the slurry tank through the filter press. Times typically range from 15 to 45 minutes, with 20 to 30 minutes being most common. In batch systems, contact time tends to be longer due to the sequential nature of the batch process. The positive effect of increased contact time is that it may improve bleached color and chlorophyll removal. Adsorption generally occurs exponentially and reaches a point of diminishing return after around 30 minutes.
It is generally accepted and understood that bleaching clays have a limited number of active sites or “parking places” for contaminants. The filling of these sites progresses well provided the amount of contaminant is matched by adequate pore space or volume and surface area.
As the proverbial parking garage nears capacity, the level of residual contaminants is so low that it is not worth spending additional production time to find a place to park the remaining residual contaminants. In contrast, excessive contact times may result in darkening of the deodorized oil (see Fig. 10) as expressed by its red color, which is most likely due to oxidation and reduction reactions that are known to occur on the active surface of the bleaching clay.
Figure 10. Effect of contact time on RBD color in palm oil.
Oil moisture content
Oil moisture typically ranges from ≤0.05 weight % in vacuum dried oil to ~0.35 weight % in oil coming directly from a water washing centrifuge into the bleaching process. Optimizing the moisture content will improve chlorophyll and phosphorus removal (see Fig. 11-13). Moisture beneficiation is, however, not guaranteed; it depends on the absolute pressure applied by the vacuum drier in use, the bleaching temperature, the level of contaminants in the oil and the nature of the bleaching clay. Bleaching at higher temperatures with higher capacity vacuum driers and higher clay levels allows bleaching operations to process oils with as high as 0.5% moisture without problems.
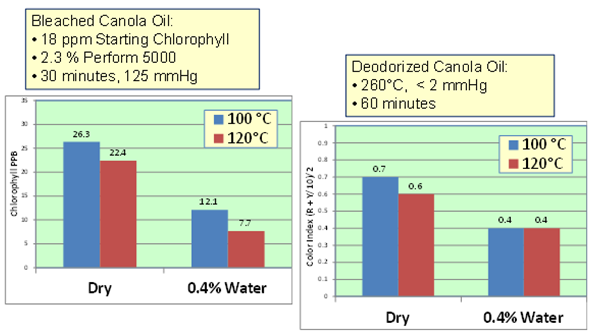
To reiterate, the optimal level of starting moisture in oil to improve bleaching is not fixed. For example, under lab conditions with 200 g oil, for 30 minutes and an adequate vacuum (125 mm Hg), it has been established that bleaching with 0.4 weight % moisture produced better chlorophyll and RBD color results than bleaching with moisture level <0.05 weight % (Fig. 11). It has also been found that a starting oil moisture content of 0.5 weight % in crude palm oil before the oil is subjected to dry-degumming (15 minutes, 85°C, atmospheric pressure) and bleaching conditions (30 minutes, 95°C, 125 mm Hg) is more favorable.
Figure 11. Optimal moisture effect in lab bleaching of canola oil: with respect to temperature, chlorophyll reduction and deodorized oil color.
When simulating plant conditions in the laboratory by using a longer contact time, it has been established that the optimal moisture is around 0.2-0.3 weight % moisture (Fig. 12). Optimal moisture levels are typically less than equal weight of the bleaching clay dosage and more likely to be around 0.2 to 0.3 weight % [11-13].
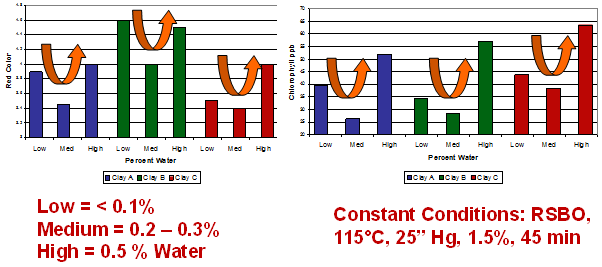
Figure 12. Oil moisture effects: in bleaching refined (neutralized) soybean oil (RSBO) at 115°C, with 1.5% clay dosage, under 125 mmHg vacuum (25 inches Hg absolute pressure) for 45 minutes.
In the presence of excess moisture, above saturation point of water with free water present in oil (<0.15 weight %), low temperatures (<20°C above the melting point) and lack of a good vacuum, bleaching efficiency can and most likely will decrease due to the slaking effect of water on the aggregate structure of the clay particles when water is in excess. Slaking of clay in this situation is the result of the wetting action on the clay granule causing it to have characteristics of “mud”.
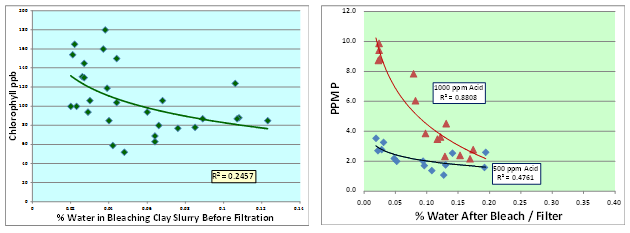
At water levels in the oil greater than saturation, water is in finely dispersed form or excess, readily available for the clay particles and all the adsorbed/hydrophilic contaminants (e.g. soaps, phospholipids) to lock on to. By lowering the water concentration in the oil below the saturation point (somewhere between 0.06% and 0.1% depending on temperature and level of contaminants), the water becomes fully dissolved in the oil and less likely to cause the clay to slake and plug the filter press. Recent work in studying moisture effects is presented in Figure 13, where it has been established that a significant relationship exists between moisture beneficiation (in both canola and dry-degummed/bleached palm oil) and moisture levels by following the post-bleached pre-filtered oil moistures (in both cases n < 30 bleach runs).
Figure 13. Moisture effects — recent work in canola (chlorophyll) and dry-degummed palm oils (phosphorus) [10].
Vacuum
Bleaching efficiency improves when operating pressure in the bleacher is run between 50 to 125 mmHg (absolute). Reduced pressure allows for a smooth water evaporation rate resulting in increased efficiency for removal of phospholipids, chlorophylloids, and some red pigments. Reduced pressure also minimizes interaction of oil and air resulting in lower peroxide values, anisidine values, and bleached oil color.
Bleaching efficiency is highly dependent on the interaction between vacuum, moisture and temperature. Bleaching clay has an attraction/affinity for polar compounds including water. Maintaining adequate moisture levels in the bleacher throughout the bleaching process affords better removal of soaps, phospholipids and chlorophyll due to bleaching clay’s affinity for these compounds. More specifically, phospholipids and soaps are more readily removed from fats and oils when properly hydrated.
The absolute pressure of the vacuum works in conjunction with temperature to drive moisture out of the oil. Depending on the incoming moisture levels, too strong a vacuum can be detrimental for bleaching efficacy in that it will pull moisture out of the system too quickly and drive moisture to below the optimal range (0.06 to 0.1%) to reap its benefits (reportedly as much as 30% improved activity). An indirect way to determine if moisture levels are in balance with vacuum and temperature is to monitor the moisture content of the oil coming out of the bleaching filter. The process is deemed optimal when the moisture content of the oil leaving the filter press approaches 0.05 weight % from the high side of the optimal range.
Filtration
The filterability of sorptive minerals, including clay minerals and diatomaceous earths (DE), depends on the natural (or created) porosity, the particle size distribution (PSD) of the product, and the type of filtering aids (DE, cellulose fibers, paper, etc.) and equipment employed. In general, the flow of oil through a given sorbent bed can be improved by increasing the particle size and/or decreasing the range of the PSD. Permeability of the filter bed also depends upon the interaction between the particles in the system with respect to the PSD of the bleaching sorbent, the amount of filter aid employed as pre-coat, and the mesh size of the filter media (paper, screens, cloth).
There is a trade-off, however, between permeability and bleaching activity. Activity for a sorbent at a given activation level generally correlates with its PSD in that activity increases as the average particle size decreases; and vice versa. For this reason, bleaching sorbents are offered with various PSDs and activities to allow the operator to achieve the best match with the equipment employed for optimum balance between throughput and activity.
Troubleshooting
One of the difficult tasks for a refinery manager is to manage the occasional refinery-upset situation. Such events demand a good understanding of the variables that are known to drive the bleaching process as discussed in the text above. When bleaching performance deteriorates, the need for troubleshooting, to sort out the symptoms from the root cause of the problem, is the greatest (Figs. 14, 15).
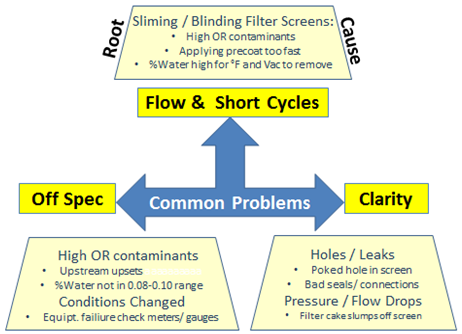
Figure 14. Bleaching operations: common problems and root causes.
In an upset situation the easiest and usually the first factor a bleaching operator targets is the bleaching clay. In this situation the typical response would be to increase clay dosage until the oil quality returns to being within specifications. However, in many cases loss in bleaching efficiency is due to other factors than the bleaching clay (Fig. 15). The bleaching clay, for the most part, is one of the most stable variables in the refining process.
Figure 15. Factors that can affect the adsorption process.
Summary
To the untrained operator, the bleaching process is a simple operation that works merely to improve oil quality through the interaction of oil and sorbent. However, this interaction is far from simple and depends on the outcome of a multitude of reactions - most of which work towards improving oil quality. Adjustment of any parameter, before and during the bleaching process, that enhances the probability of clay and contaminant interacting will drive the kinetics of adsorption in an optimal direction.
Optimization of the bleaching process requires an effort to maximize the interactions that promote quality with minimal detriment to the oil. So many variables can influence the outcomethat, despite the science behind it, we still consider it an art to find that optimal point under each set of circumstances that a plant can present.
References
- Berbesi, R. Practical optimization of bleaching process. AOCS Short Course, Long Beach, CA (2011).
- Zschau, W. Bleaching. In: Introduction to Fats and Oils Technology. pp.158-178 (R.D. O'Brien, W.E. Farr, and P.J. Wan (eds.), AOCS Press, Champaign IL) (2000).
- Patterson, H.B.W. Adsorption. In: Bleaching and Purifying Fats and Oils. Theory and Practice. pp.53-67 (G.R. List (ed.), AOCS Press, Urbana, IL) (2009).
- Ramli, M.R., Siew, W.-L., Ibrahim, N.A., Hussein, R., Kuntom, A., Razak, R.A.A. and Nesaretnam, K. Effects of degumming and bleaching on 3-MPCD esters formation during physical refining. J. Am. Oil Chem. Soc., 88, 1839-1844 (2011) (DOI: 10.1007/s11746-011-1858-0).
- De Greyt, W.F.J. Review on 3-MCPD and glycidyl esters in vegetable oils and fats. Accessible at: aocs.files.cms-plus.com/ResourcesPDF/MCPD-GE-mitigation-AOCS-2012(DGW)-final.pdf.
- Malaysian Palm Oil Board MPOB statement on 3-MCPD esters, Accessible at: www.mpob.gov.my/en/component/content/article/153-demo-content/731-mpob-statement-on-3-mpcd-esters.
- Transfeld, P., Schneider, M. and Börner, G. (Öhmi Forschung und Ingenieurtechnik GmbH), Method and apparatus for adsorptive cleaning of substances. US Patent 5,753,103 (1998).
- Waranica, G. Double pass bleaching system. Accessible at: www.crowniron.com/userimages/BleDoubleN1.pdf.
- Official Methods and Recommended Practices of the AOCS, 6th Edition, 2nd Printing (AOCS Press, Urbana, IL) (2011).
- Brooks, D.D. The effect of bleached oil moisture in bleaching dry & semi-dry degummed crude palm oil. Paper presented at the LAOCS Conference, Cartagena, Colombia (2011).
- Bailey's Industrial Oil & Fat Products (Y.H. Hui (ed.), John Wiley & Sons, Inc., New York) (1996).
- Gupta, M.K. Practical Guide to Vegetable Oil Processing (AOCS Press, Urbana, IL) (2008).
- Canola, Chemistry, Production, Processing, and Utilization (J.K. Daun, N.A.M. Eskin, and D. Hickling (eds.), AOCS Press, Urbana, IL) (2011).
In This Section
- Marine Oils
- Animal Fats
- Olive Oil
- Palm Oil
- Seed Preparation
- Expanding and Expelling
- Solvent Extraction
- Meal Desolventizing, Toasting, Drying and Cooling
- Introduction to Degumming
- Chemical Degumming
- Enzymatic Degumming
- Alkali Refining
- Optimization of Bleaching Process
- Silica Hydrogel and its Use in Edible Oil Processing
- Deodorization
- Hydrogenation Mechanism
- Chemical Interesterification
- Enzymatic Interesterification
- Solvent Fractionation
- Dry Fractionation
- Hydrogenation in Practice