Deodorization
The Author: Wim De Greyt, R&D Manager, Desmet Ballestra, Zaventem, Belgium.
1. Introduction
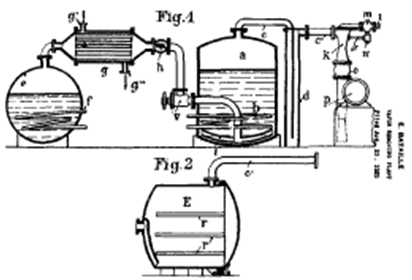
In the early days of the edible oil processing industry, in the first half of the 19th century, there was little or no need for refining. Food fats (e.g. lard, olive oil, milk fat, etc.) were mostly consumed unrefined and their typical flavour was even an attractive characteristic [1]. It was the growth of the margarine industry in Europe at the end of the 19th century that resulted in the development of the edible oil deodorization process (Table 1). At first, industrial deodorizers were mostly batch-type with the ‘Bataille’ and ‘Lurgi’ deodorizers being the most widely used in Europe [1,2] (Fig. 1). These deodorizers operated under vacuum (to facilitate stripping and protect the oil against oxidation) and also used superheated steam as a stripping agent (to avoid hydrolysis).
| Table 1. First developments of the edible oil deodorization process (Europe and USA) [1] | |||
| Inventor | Country | Year | Development |
| Rocca | France | 1900 | Continuous deodorizer at atmospheric pressure |
| Bataille | France | 1914 | Batch deodorizer under vacuum and with superheating of the stripping steam |
| Gensecke and Brucke | Germany | 1916 | First ‘Lurgi’ deodorizer, similar to the Bataille deodorizer but consisting of two vessels and also with internal baffle construction for better oil/steam contact |
| De Bruyn | Belgium | 1900 | Continuous countercurrent column deodorizer at atmospheric pressure. Column filled with screen-plates |
| Eckstein | USA | 1891 | Large volume batch deodorizer (5-15 metric tonnes) running at atmospheric pressure but without steam superheating |
| Wesson | USA | 1900 | First deodorizer in US running under vacuum (never patented) |
Figure 1. Bataille High Vacuum Batch Deodorizer (source: [1]).
In the USA, it was Eckstein who developed the first industrial deodorizer. In 1891, he demonstrated that the flavour of alkali-refined cottonseed oil could be greatly improved by blowing live steam through the oil at high temperature (160-175°C). The most successful American deodorizing process was that of Wesson, which was introduced in 1900 by the Southern Cotton Oil Company. The process was not patented and kept secret for a time but it was probably the first vacuum deodorizing process in the US. The quality of Wesson’s deodorized oil was for many decades a standard for edible oils throughout the world [1].
Over the years, deodorization gradually evolved from a ‘simple’ process to remove off-flavors to a crucial unit operation with a big impact on the refined oil quality. In current edible oil refining, deodorization is also the process in which free (nonesterifed) fatty acids (in the case of physical refining) and volatile contaminants are stripped and unwanted color pigments are degraded (heat bleaching).
Although the principle of the process has not changed much since its first application, the deodorizing technology itself has changed significantly. It has been steadily improved to meet the need for ever more efficient processing (lower operating cost, higher refined oil yield and better valorization of side streams). More recently, increasing attention to the (nutritional) quality of food oils and fats has had an impact on the deodorizing process conditions.
2. Deodorization principle
Deodorization is actually a stripping process in which a given amount of a stripping agent (usually steam) is passed for a given period of time through hot oil at a low pressure. Hence, it is mainly a physical process in which various volatile components are removed. However, since it is usually carried out at high temperature (>200°C), some chemical and thermal effects may take place as well.
Vacuum stripping of volatile components
Theoretical aspects of vacuum stripping have been described extensively by many authors [3-5]. Stripping of a given volatile component from the oil is determined by its intrinsic volatility (vapor pressure curve) and the deodorizing conditions applied (temperature, pressure and amount of sparge steam). For a batch and cross-flow deodorization process, the stripping effect is described by the following mathematical equation:
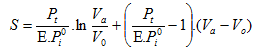 |
[1] |
- with S = total moles of steam or any other stripping agent per mole of oil (to express the amount of steam as a percentage of the oil, the factor S has to be multiplied by a factor of 2.); Pt = total pressure of the gas phase = system pressure; Pi0 = Vapor pressure of a given fatty acid i; E = vaporization efficiency; Va = initial amount of the volatile component in the oil (moles), V0 = final amount of the volatile component in the oil (moles).
Other, similar equations have been derived for counter- and co-current deodorization [4].
From equation (1), it can be concluded that the amount of sparge steam required for the stripping of a given volatile component (e.g. free fatty acids) is :
- Directly proportional to the absolute pressure in the deodorizer;
- Inversely proportional to the vapour pressure of the volatile component;
- Inversely proportional to the overall vaporization efficiency E
From the factor (ln Va/V0), it can also be derived that :
- It is impossible to eliminate all volatile components during deodorization;
- Halving the concentration of a given volatile component requires the same amount of stripping steam, irrespective of its absolute concentration
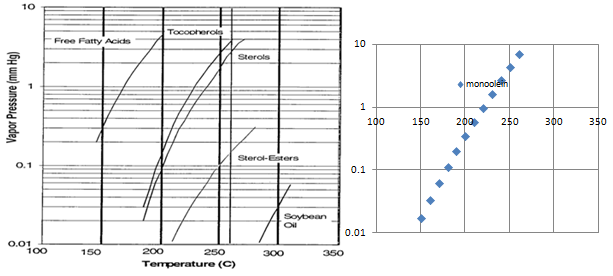
Edible oils contain various components, each with its specific volatility (Fig. 2). In physical refining, it is mainly free fatty acids (FFA) that need to be stripped. Apart from FFA, other volatile components, either valuable (tocopherols, sterols, etc.) or unwanted (off-flavors, pesticide residues, light polycyclic aromatic hydrocarbons, dioxins, etc.), are also removed during deodorization.
Figure 2. Vapor pressure-temperature relationship for different components in edible oils (♦ : curve for monoolein).
The vaporization efficiency E in equation (1) is a deodorizer design-specific factor. It should be seen as a measure of how saturated with volatile components the stripping agent (steam) becomes during its contact with the oil. In an ideal (theoretical) case, E = 1, but industrial deodorizers usually have a vaporization efficiency of 0.3-0.7, depending on their design (steam injection geometry, depth of oil layer, elimination of reflux, etc.).
Thermal effects
Another objective of deodorization is the thermal destruction of flavor precursors and heat-sensitive color pigments. The latter effect is called ‘heat bleaching’ and it is most pronounced during deodorization/steam refining of palm oil, where the thermal breakdown of carotenes is targeted. Heat degradation of carotene is very slow at 210°C, but takes only a few minutes at T > 260°C. This is one reason why palm oil is typically deodorized at 260°C.
However, there is a general trend to lower the ‘heat load’ (residence time at high temperature) used during deodorization. This evolution towards milder process conditions is caused by the increasing awareness of the potentially harmful health effects of thermal degradation products (trans fatty acids, polymeric triglycerides and glycidyl esters) that can be formed during deodorization. In addition, there is the desire for maximum retention of the natural oil characteristics.
Effective deodorization: combination of stripping and thermal effect
Perfect deodorization is a complex process which includes the removal of volatile off-flavors already present in the bleached oil as well as the off-flavors that are formed during thermal degradation of higher molecular weight flavor precursors. Removal of the first group is similar to FFA stripping and can be achieved in a short time. Longer deodorization time is required to convert non-volatile flavor precursors into volatile off-flavors that can be stripped from the oil.
In practice, this means that time is an important process parameter in obtaining a refined oil with a bland and stable taste. If the deodorization time is too short, some flavor precursors will stay in the deodorized oil, resulting in the development of off-flavors during storage or usage. This phenomenon, which is known as ‘flavor reversion’, is well known but at the same time still poorly understood.
3. Deodorized oil quality
Deodorized oil quality is evaluated primarily by traditional quality parameters such as a low residual FFA content, a high oxidative stability, a light color and a bland odor and taste. In addition, high-quality food oils need to contain low trans fatty acid (TFA) levels, high amounts of natural antioxidants (tocopherols), low levels of polymeric and oxidized triglycerides and no contaminants or degradation products. Refining targets for these minor components are given in Table 2.
| Table 2. Refining targets for various minor components in edible oils | |
| Minor component | Refining target (concentration in fully refined oil) |
| trans Fatty acids | <1.5% for oils rich in linolenic acid (soybean oil, rapeseed oil, canola, etc.) <1.0% for other vegetable oils (corn oil, sunflower oil, etc.) |
| Tocopherols | min. 500 ppm (to guarantee good oxidative stability) |
| Polycyclic aromatic hydrocarbons (PAH) | New EU regulation 835/2011 in force since September 1, 2012 <2 ppb BaP and <10 ppb PAH4 for most food oils; <2 ppb BaP and <20 ppb PAH4 for refined coconut oil; <5 ppb BaP and <30 ppb PAH4 for cocoa butter BaP = benzo(a)pyrene PAH4 = sum of BaP, benz(a)anthracene, benzo(b)fluoranthene and chrysene |
|
Dioxins and PCB |
New EU regulation 1259/2011 in force since January 1, 2012 For refined vegetable oils: <0.75 ppt WHO-TEQ (dioxins) and <1.25 WHO-TEQ (dioxins + dioxin-like PCB) For marine oils : < 1.75 ppt WHO-TEQ (dioxins) and < 6 WHO-TEQ (dioxins + dioxin-like PCB) WHO-TEQ = World Health Organization toxic equivalent PCB = polychlorinated biphenyls ppt = parts per trillion |
| 3-MCPD + GE1 | No legal specifications yet; only trade specifications which are especially challenging for palm oil <2 ppm for refined oils for use in standard food applications; <0.5 ppm for refined (palm) oil for use in infant food. |
1 3-Monochloropropane-1,2-diol and glycidyl esters |
|
4. Deodorizing process conditions
The deodorization process is fully determined by four process parameters : (1) temperature, (2) time, (3) pressure and (4) amount of stripping steam. The effects of process conditions on the standard quality parameters and the nutritional quality of the refined oil are well understood and are described in the literature (Table 3) [4,5].
| Table 3. Effect of process variables on deodorized oil quality | ||||
| Quality parameter | Temperature | Time | Pressure | Steam |
| Taste | + | ++ | + | ++ |
| Color (heat bleach) | ++ | + | - | - |
| FFA stripping | ++ | - | ++ | + |
| trans Fatty acid formation | ++ | ++ | - | - |
| Tocopherol/sterol stripping | ++ | - | ++ | + |
| Contaminant removal1 | + | - | ++ | + |
| Glycidyl ester formation | ++ | ++ | - | - |
1 Pesticides, PAH, dioxins; - : little or no effect, + : significant effect, ++ : large effect |
||||
Optimal process parameters depend on the type of oil (bleached and refined oil specifications) and the refining process applied (chemical or physical), but the limitations of available deodorizing equipment and the need to minimize operating costs are also determining factors. The typical range of the different deodorizing process parameters is given in Table 4.
| Table 4. Typical process conditions for edible oil deodorization | ||
| Parameter | Range | Comment |
| Temperature | 160-260°C | Lower temperature (<200°C) for heat- sensitive oils (e.g. cocoa butter, fish oil) to avoid too much degradation of omega-3 fatty acids (fish oil) and negative effects on crystallisation characteristics (cocoa butter) -Higher temperature (260°C) for FFA stripping/heat bleaching (e.g. physical refining of palm oil) Trend towards lower deodorizing temperature (230-240°C) |
| Time | 5 min – 4 hr | FFA stripping (with packed column): 5 min (no deodorization) Deodorization of soybean/canola oil: 20-90 min Full deodorization of fish oil: 2-4 hr |
| Pressure | 1.5 – 5 mbar | Most common range : 2-4 mbar Low pressure required for stripping of FFA and volatile contaminants (pesticides, light PAH, etc.) Trend towards lower deodorizing pressure. This allows same stripping efficiency at lower temperature or with less stripping agent Higher cost to create lower deodorizing pressure |
| Stripping steam | 0.5 – 3% | Depending on type of oil and refining mode Steam is the most commonly used stripping agent (efficient – lowest cost) Stripping with nitrogen is not applied industrially |
5. Deodorizing process and deodorizer technology
Deodorization is a multistep process comprising deaeration, multistage heating, deodorization-deacidification, and multistage cooling of the oil (Fig. 3).
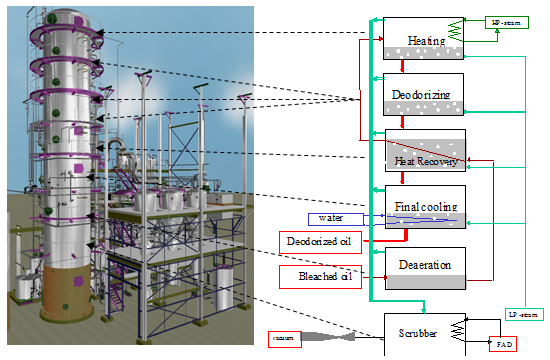
Figure 3. Schematic presentation of the different stages of the deodorizing process.
Oil deaeration
Degummed, bleached oil is deaerated prior to heating to deodorizing temperature to avoid oxidation and polymerization. It is accomplished in a separate external vessel connected to the vacuum system of the bleacher (50 mbar) or, at even lower pressure, in an integrated compartment of the deodorizer (Fig. 3). Some refiners add a bit of sparge steam to improve deaeration.
Heating and cooling
Heating of the oil is usually accomplished in two or more stages. To minimize the net energy cost, bleached oil is first pre-heated in one or two stages in a heat exchange device with either hot deodorized oil or steam.
The highest energy recovery (up to 85%) can be achieved in continuous deodorizers in which bleached oil is pre-heated indirectly with hot deodorized oil. This heat recovery usually takes place in a heat recovery compartment of the deodorizer, but it can also be realized in a separate, external heat exchanger. Both options have their pros and cons. External heat exchangers result in a high heat recovery and provide easier access for cleaning. On the other hand, heat exchange in the deodorizer ensures less product intermixing and less risk of fouling and it also takes place under vacuum.
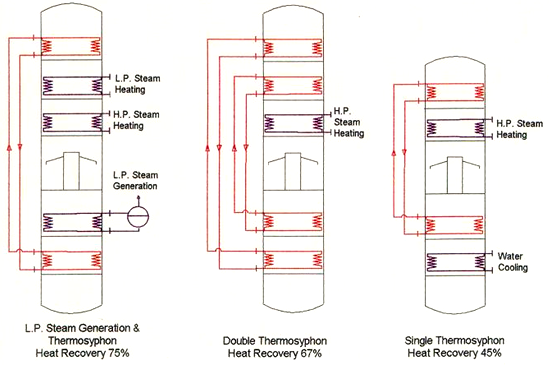
The thermosiphon system is a special method of heat recovery that is used in semicontinuous deodorizers (Fig. 4). Steam produced in the oil cooling section flows in a closed loop to the oil pre-heating section. It will condense there and the water flows back to the cooling section. In this way, a heat recovery of 45-75% can be achieved, depending on the design of the thermosiphon system (single or double loop, with or without generation of low-pressure steam).
Figure 4. Thermosiphon heat recovery options in a semicontinuous deodorizer.
Final cooling of the oil can be done under vacuum or under pressure. Which practice is the best has always been a matter of discussion.
Deodorization-deacidification
Since the concentration of most volatile components in edible oils is quite low, a stripping agent must be injected during deodorization. For economic reasons, steam is the most commonly used stripping agent, but the use of nitrogen has also been studied extensively. Nitrogen is an inert gas and theoretically, its use will result in lower losses (no hydrolysis) and also a higher quality of the deodorizer distillate. However, in industrial practice, nitrogen is not used primarily because it is a noncondensable gas. This makes the required vacuum system much more expensive than the use of steam, which is condensable.
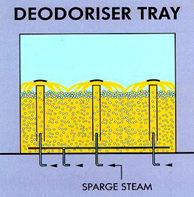
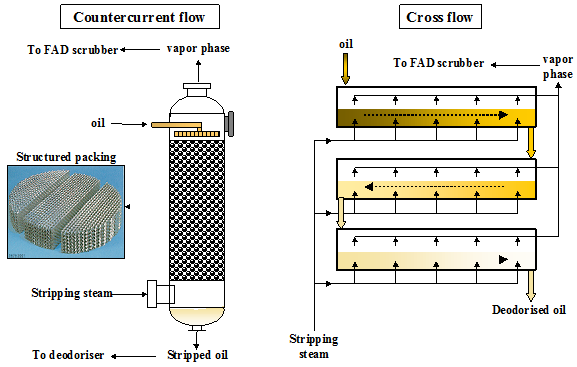
Most semi- and continuous deodorizers are so-called tray deodorizers which operate according to the cross-flow principle. Deodorization-deacidification is accomplished in a number of compartments (trays) where stripping steam is introduced into the oil through special sparge coils with very fine holes or by steam lift pumps. The latter give good agitation with continuous refreshing of the oil in the top layer (where deodorization effectively takes place), thereby ensuring a high overall deodorization efficiency (Fig. 5). A minimal oil layer depth (more than 0.8 m) is required to allow good operation of the steam lift pump.
Figure 5. Principle of a steam lift pump for the introduction of sparge steam.
The stripping efficiency of a deodorizer can be further improved by incorporating a packed column stripper. Such a stripper is filled with a structured packing with a high surface area (250-350 m2/m3) (Fig. 6). The countercurrent contact of oil and stripping steam over the structured packing results in very efficient stripping in a short contact time.
Figure 6. Principle of countercurrent and cross flow stripping.
Packed column strippers have been applied in edible oil deodorization for many decades. They are often installed to increase the capacity of existing deodorizers. It is a very efficient device for the stripping of FFA or volatile contaminants (pesticides, PCB, light PAH, etc.). The short residence time makes it especially suitable for the stripping of heat-sensitive oils (e.g. algae oil, fish oil, cocoa butter, etc.). At the same time, this short residence time will not give much heat bleaching nor complete deodorization. For this purpose, an additional retention vessel has to be provided before or after the packed column.
Vapour scrubbing systems
The vapors leaving the deodorizer consist of steam, volatile components (fatty acids, sterols, tocopherols, contaminants, etc.), minor amounts of mechanically entrained neutral oil (mono-, di- and triacylglycerols) and some noncondensables (e.g. air, etc.). Condensation of the volatile components is achieved in a scrubber and results in a by-product called deodorizer distillate.
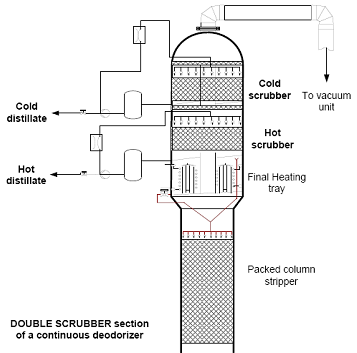
Condensation is achieved by creating a very good contact between the hot vapour phase and the cold deodorizer distillate that is partially recirculating over the scrubber. In practice, this is done by a series of sprayers built in the duct or on a packed bed of limited height in the scrubber vessel itself. An additional demister is usually installed ahead of the vacuum unit to minimize carryover of fatty matter to the barometric condenser water (Fig. 7).
Figure 7. Double scrubber for selective condensation of FFA and tocopherols (courtesy of Desmet Ballestra - Source: [6]).
A well-designed scrubber should combine maximum condensation and recovery of fatty matter from the vapor phase with a minimum pressure drop (<1 mbar, preferably <0.5 mbar).
Most deodorizers have a single scrubber from which a single deodorizer distillate is collected. The amount and composition and, hence, also the value of this side-stream are determined by a number of factors, including the processed oil composition, the refining mode employed (chemical or physical) and also the deodorizing conditions (Table 5). Deodorizer distillate from physical refining has a very high FFA content (>85%) and is mostly used for technical applications (soap production, oleochemistry). Recently, deodorizer distillate from palm oil refining (PFAD) is also used as feedstock for biodiesel production. Deodorizer distillate from chemical refining may have a higher value because of the higher concentration of valuable minor components (tocopherols, sterols, etc.). The value of deodorizer distillate from chemical refining of soybean oil is especially high. Depending on the price of ‘natural’ tocopherols, it can vary between $15-25 US/tonne of deodorized oil [6].
Soya oil
| Table 5. Typical composition of deodorizer distillates from vegetable oil refining | ||||
| Chemical refining | Physical refining | |||
| Soya oil | Canola oil | Sunflower oil | Palm oil | |
| FFA (%) | 25-40 | 85-90 | ||
| Tocopherols (%) | 5-20 | 8 | 5-10 | 0.15-0.30 |
| Sterols (%) | 6-23 | 7 | 12-14 | 0.2-0.4 |
| Squalene (%) | 0.1-3.0 | - | 0.5 | 0.5-1.0 |
| Neutral oil (%)1 | 10-50 | 6-9 | ||
| Distillate flow (%)2 | 0.3-1.0 | |||
1Neutral oil = mono- + di- + triglycerides; 2expressed on bleached oil. |
||||
In the case of physical refining, a deodorizer distillate fraction rich in tocopherols/sterols can only be obtained with a double scrubber (Fig. 7). The vapor phase is first partially condensed at a higher temperature. This gives a so-called ‘hot distillate’ in which the least volatile components (e.g. tocopherols/sterols) are concentrated. Complete condensation of the remaining more volatile components (mainly FFA) is then achieved in the second, cold scrubber. Provided that the condensation temperatures of the hot and cold scrubber are properly set, this procedure gives a very good separation between FFA and tocopherols.
Vacuum systems
The vacuum in the deodorizer is usually created by a combination of steam ejectors (boosters), vapor condensers and mechanical (liquid-ring) vacuum pumps. These quite robust systems typically reach pressures in the deodorizer between 2.5 and 5 mbar but motive steam consumption is high (up to 85% of the total steam consumption). Motive steam consumption can be significantly reduced (by a factor of 2.5-3) by cooling the barometric condenser water. However, the benefit of a lower motive steam consumption must be weighed against the extra chilling capacity required (higher electricity consumption). Another benefit from using a chilled water barometric vacuum system is a better condensation of the volatile matter, which also gives a lower pressure in the deodorizer (e.g. 1.5 mbar).
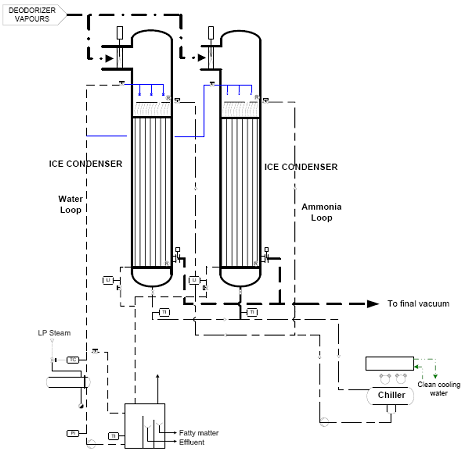
These classical vacuum systems are increasingly being replaced by dry (ice) condensing systems. With such systems, the stripping steam is condensed on surface condensers operating alternately at very low temperature (-30°C). The efficient solidification of steam and other volatile matter will give a very low pressure in the deodorizer (<1.5 mbar) and will also strongly reduce odor emission. As with the chilled water barometric vacuum system, dry ice condensers require extra electrical energy. Commercially available systems consist of two or more freeze condensers with horizontally or vertically orientated straight tubes, a refrigeration plant for the generation of the cold refrigerant which is evaporated in the tubes and a vessel for defrosting and cleaning of the tubes after a certain period of freezing (Fig. 8).
Figure 8. Typical process flow diagram of an ice condensing system (courtesy of Desmet Ballestra – source [6]).
6. Industrial deodorizers
Edible oil deodorization is performed industrially in different ways (continuous, semicontinuous or batchwise) with various configurations of deodorizers (horizontal or vertical vessels, tray-type or packed columns). Selection of the most appropriate process technology is mainly determined by the total plant capacity and the number of feedstock changes.
Batch deodorizers
Overall, batch deodorization has become less attractive because of its higher operating cost (higher steam consumption, low heat recovery) and longer processing time. However, for small-capacity plants (<50 tons/day) or plants that process smaller batches of varying quality (e.g. fish oil refiners), a batch deodorizer is still the best option. Apart from the lower capital cost, the main advantages of a batch deodorizer are the higher flexibility (process parameters can easily be adjusted according to the incoming oil quality) and the minimal intermixing between two consecutive batches.
Semicontinuous deodorizers
Semicontinuous deodorizers are basically batch systems designed for larger capacities. Their main application is in plants with frequent feedstock changes of oils sensitive to intermixing (e.g. plants producing margarine fats and shortenings). Heat recovery is effected by means of indirect economizers (e.g. thermosiphon systems, Fig. 4) which recover more heat than can be achieved in a batch deodorizer. The lower intermixing and the shorter time for feedstock changes are the main advantages of semicontinuous deodorizers over continuous deodorizers.
Continuous deodorizers
Continuous deodorizers are the best option for high-capacity plants running on a single feedstock (which is how most refining plants operate nowadays). The main advantages are the moderate investment costs, potentially high heat recovery and easy maintenance. An overview of the various configurations can be found in the literature [5,6].
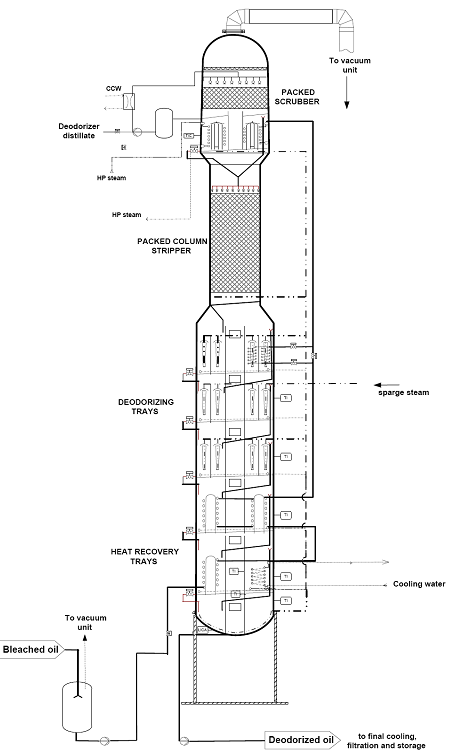
Vertical tray-type deodorizers are the most commonly used type of continuous deodorizers. Their design is based on a series of trays (compartments) stacked vertically in a cylindrical shell with each tray designed for a specific task. All processes (heat recovery, final heating and cooling, deodorization) are combined in one single vessel. This single vessel concept allows an easier and less costly installation and maintenance and also gives a lower risk of unwanted air leakages.
Most edible oils are deodorized at high temperature (230-260°C), but there is a growing demand from oil processors to lower the heat load (residence time at higher temperature) during deodorization. This is especially required to minimize thermal degradation reactions (e.g. formation of trans fatty acids in soybean and canola oil, formation of glycidyl esters in palm oil). These negative thermal effects can be minimized by using packed columns or dual-temperature deodorizers (Fig. 9). These deodorizers operate at two different temperatures in order to reach the best compromise between required residence time for actual deodorization (longer time at lower temperature) and heat bleaching and stripping of volatile components (shorter time at higher temperature). The dual temperature concept has been successfully introduced on an industrial scale. Both the low/high temperature and the high/lower temperature concept can be applied.
Figure 9. Dual temperature with packed column.
7. Future trends
In their ‘historical sketch’ of edible oil deodorization, Lee and King stated in 1937 that the ‘current trend (in edible oil deodorization) is toward continuous processes with automatic control’ [1]. Nowadays, new developments in deodorizing technology are driven by the continuous need for more efficient processing (lower operating cost, higher refined oil yield and better valorization of side streams) and the increased attention to the (nutritional) quality of food oils (Table 6).
| Table 6. Trends and developments in edible oil deodorization. | |
| Trend | Development |
| Higher capacities | 1500 metric tons per day deodorizers become standard |
| Higher energy efficiency | Improved heat recovery |
| Higher stripping efficiency | Improved tray design and introduction of packed columns |
| Lower neutral oil losses | Improved scrubber design |
| Lower heat load | Application of dual temperature deodorization and use of a packed column |
| Lower pressure | Ice condensing vacuum systems: closed loop with chilled water |
| Higher distillate value | Application of double scrubber |
Fixed costs are primarily reduced by installing higher-capacity (continuous) deodorizers. Today, deodorizers with a capacity of more than 1500 tonnes per day have become more or less standard, especially for the deodorization of commodity oils (e.g. palm oil, soybean oil, etc.). Variable processing costs are determined by the energy consumption for the heating of the oil, generation of the vacuum and production of stripping steam.
Process conditions are optimized to guarantee a good nutritional quality, meaning minimum thermal degradation and maximum stripping of contaminants. This can be achieved by applying dual temperature deodorization and/or including a packed column stripper. A further reduction of the heat load can be achieved by the implementation of more powerful vacuum systems (chilled barometric vacuum system or dry ice condensing).
References
- Lee, A.P. and King, W.G. Edible oil deodorizing equipment and methods: A short historical sketch. Oil & Soap, 14, 263-269 (1937).
- Fritsch, J. Raffinage des huiles. In: Fabrication et Raffinage des Huiles Végétales, pp.686 (Amédée Legrans, Paris) (1931).
- Norris, F.A. Deodorization. In: Bailey's Industrial Oil and Fat Products, 4th edition, Vol. 3, pp. 127-156 (T.H. Applewhite (ed.), John Wiley & Sons, (Hoboken, NJ) (1985).
- Dijkstra, A.J. Vacuum stripping of oils and fats. In: The Lipid Handbook, 3rd Edition, pp. 235-253 (F.D. Gunstone, J.L. Harwood and A.J. Dijkstra (eds.), Taylor & Francis Group LLC, Boca Raton, FL) (2007).
- De Greyt, W.F.J. and Kellens, M.J. Deodorization. In: Bailey's Industrial Oil and Fat Products, 6th edition, Vol. 5, pp. 341-338 (F. Shahidi (ed.), John Wiley & Sons, Hoboken, NJ) (2005).
- De Greyt, W.F.J. Edible oil refining: Current and future technologies. In: Edible Oil Processing, pp.127-151 (W. Hamm, R.J. Hamilton and G.H. Calliauw (eds.), John Wiley & Sons, Chichester) (2013).
In This Section
- Marine Oils
- Animal Fats
- Olive Oil
- Palm Oil
- Seed Preparation
- Expanding and Expelling
- Solvent Extraction
- Meal Desolventizing, Toasting, Drying and Cooling
- Introduction to Degumming
- Chemical Degumming
- Enzymatic Degumming
- Alkali Refining
- Optimization of Bleaching Process
- Silica Hydrogel and its Use in Edible Oil Processing
- Deodorization
- Hydrogenation Mechanism
- Chemical Interesterification
- Enzymatic Interesterification
- Solvent Fractionation
- Dry Fractionation
- Hydrogenation in Practice