Silica Hydrogel and its Use in Edible Oil Processing
The Author: Tony Nock, PQ Corporation
Introduction
Crude triglyceride oils and fats from a wide range of vegetable or animal sources undergo a refining process in order to remove contaminants which may negatively impact the appearance, taste or stability of the final product. Such contaminants include free fatty acids, trace metals, phosholipids, color bodies and oxidation products.
The Use of Adsorbents in Edible Oil Refining
An important step in the refining process involves the use of an adsorbent material to remove contaminants from the oil. The adsorbent is dosed into the oil as a free-flowing powder. The mixture is then agitated at the required temperature for a period of time necessary to allow the contaminants to be adsorbed by the surface of the adsorbent particles. Once the contaminants are bound to the surface of the adsorbent particles, the mixture is filtered to remove the spent adsorbent. Traditionally, the step of adsorbent treatment in edible oil processing is referred to as Bleaching and involves treatment of the oil with a clay-based adsorbent called bleaching earth or bleaching clay[1-3]. As the name suggests, the primary purpose of the bleaching step is to remove color bodies from the oil, for example chlorophyll and carotene. However, the bleaching step is responsible for removal of a number of other contaminants including trace levels of phospholipids, metals and oxidation products, together with soaps which are generated upstream in the refining process.
Silica Hydrogel Production and Properties
Silica hydrogel, sometimes called aquagel[4], is so called because of its high moisture content(typically 60-65%wt). This matrix of pure silicon dioxide and water is a free-flowing finely ground white powder. These products are generally referred to as synthetic amorphous silica gels.
Silica hydrogel is produced by reacting sodium silicate solution with an acid[4]. Sodium silicate solution, commonly known as water glass, comprises a distribution of polymeric silicate anions with charge-balancing sodium cations. Addition of a mineral acid destabilizes the silicate solution forcing the silicate polymers to form colloidal silica particles, known as a silica hydrosol. The size of the colloidal particles, typically 1.5 to 2.0 nm, depends on silica concentration, electrolyte level, amount of excess acid and temperature. The highly active colloidal silica particles continue to interact to form a solid close-packed structure with water immobilized within the matrix. The rigid translucent silica hydrogel is broken into pieces and then washed to remove excess acid and salt by-product. The hydrogel is also aged at the appropriate pH to form interconnecting voids or pores within the silica particles. Once the required pore structure has been developed, the particles are then milled and classified to give carefully controlled particle size distribution with excellent filtration properties and high purity.
Benefits of Silica Hydrogel.
Synthetic amorphous silica hydrogel is ideally suited for use as an adsorbent in refining edible oils, offering a number of environmental and cost benefits[5,6]. The high moisture content trapped within the silica structure creates the ideal environment to attract polar impurities from the oil. Contaminants such as soaps, phospholipids and trace metals are drawn into the pores of the silica and adsorbed onto the silica surface which is populated with a high concentration of hydroxyl groups. The network of pores within the silica particles offers a high internal surface area to absorb the polar contaminants. Each tiny particle, typically 15 microns in size, is like a small sponge with a high adsorption capacity.
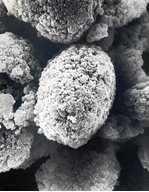
Figure 1. Electron micrograph of silica particles showing the sponge-like structure.
Physical Properties
Table 1 shows typical physical properties of a synthetic silica hydrogel (Sorbsil®R92 produced by PQ Corporation). For comparison, typical properties of a bleaching clay-based natural silicate adsorbent are also shown.
| Table 1. Typical adsorbent physical properties | ||
| Silica Hydrogel (Sorbsil® R92F) | Clay-based adsorbent | |
| Surface area, m2/g | 615 | 150 |
| Pore volume, mL/g | 1.31 | 0.51 |
| Average particle size, microns | 17.5 | 21.1 |
| Permeability, darcy | 0.11 | 0.03 |
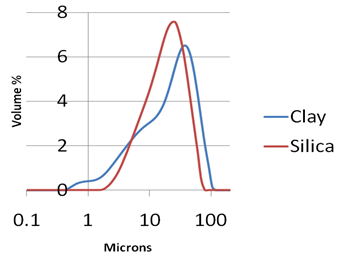
The combination of high surface area and high pore volume gives silica hydrogel superior adsorption capacity to that of the clay-based adsorbent. Average particle size measurements of the two adsorbents are similar. However, permeabilities are very different. Permeability, measured in Darcy, is a measure of the filterability of a powder. One Darcy - unit of permeability – is equivalent to the passage of one cubic centimeter of fluid of 1 centipoise viscosity flowing in one second under a pressure of one atmosphere through a porous medium of one square centimeter area and one centimeter long. It is a measure of the rate of flow of fluid through a filter bed. The larger the measured permeability value, the better the filtration properties of the powder. Permeability of the silica hydrogel is significantly better than that of the clay-based adsorbent. Comparison of the full particles size distributions for the two adsorbents (Figure 2) shows the reason for the difference in filtration properties. The graph shows the distributions of particle sizes in a slurry of each of the adsorbent powders, as measured by laser light scattering using a Malvern Mastersizer (Malvern Instruments Ltd, UK). Although the average particle size for each material is similar, the distribution of particle sizes for the silica hydrogel is narrower. In particular, the tail of fine particles seen in the clay sample is absent from the silica hydrogel powder. When the adsorbent material forms a filter cake, fine particles can fit into the voids formed between the larger particles, having the effect of plugging the filter bed and reducing the permeability. The narrow particles size distribution of the silica hydrogel maintains the void spaces between the particles in the filter cake, thereby improving filterability.
Figure 2. Particle size distribution of silica hydrogel compared with a clay-based adsorbent (measured by Malvern Mastersizer).
Chemical Properties
Because synthetic amorphous silica hydrogels are produced from carefully selected high quality raw materials they have a high degree of purity. Table 2 shows the chemical properties of a typical silica hydrogel (Sorbsil® R92 from PQ Corporation) compared with a clay-based adsorbent.
| Table 2. Typical adsorbent chemical properties | ||
| Silica Hydrogel (Sorbsil® R92F) | Clay-based adsorbent | |
|---|---|---|
| Total moisture, %wt | 59.2 | 18.3 |
| pH | 2.19 | 3.29 |
| Al content, ppm | 9.3 | 2124 |
| Fe content, ppm | 0.71 | 9926 |
The high moisture content of the silica hydrogel enhances the affinity of the silica for polar impurities in the oil, improving the purification performance of the adsorbent. The lower pH of the silica hydrogel assists the hydration and removal of some of the so called non-hydrateable phospholipids and soaps in the oil. Normally associated with trace metals magnesium and calcium, these non-hydrateable phosphatides are more easily hydrated by the lower pH silica hydrogel.
Adsorption Capacity
The combination of a number of the physical and chemical properties described above result in superior adsorption characteristics of silica hydrogels for the removal of phospholipids, trace metals and soaps from triglyceride oils.
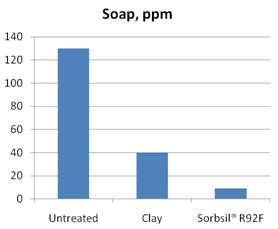
Figure 3 compares the adsorption of soap by silica hydrogel with that of a clay-based adsorbent. Caustic refined soybean oil having a residual soap level of around 130ppm was used in both tests, in each case using 0.1%wt adsorbent. After adsorptive treatment, the residual soap content of the oil is significantly lower in the case of the silica hydrogel treatment.
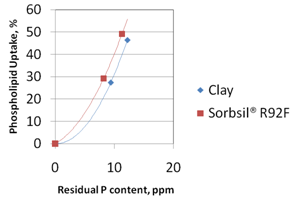
Similar results are seen for phospholipid removal in Figure 4, which shows phospholipid adsorption isotherms for the two adsorbents. These isotherms were generated by treating the same degummed oil with a range of adsorbent loadings at the same temperature. For each test, the residual phosphorus content of the oil was measured and the amount of adsorbed phospholipid (uptake) was calculated from the difference between the initial and final phosphorus content of the oil divided by the %wt adsorbent loading. The silica hydrogel has a greater phospholipid uptake compared with the clay. At low residual phosphorus levels in the oil (typically
Figure 3. Removal of soap from caustic refined soybean oil using 0.1%wt adsorbent at 80°C.
Figure 4. Phospholipid adsorption isotherms for silica hydrogel and clay-based adsorbent.
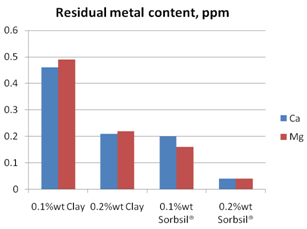
Figure 5 shows a comparison of the two adsorbents for trace metal removal, in this case magnesium and calcium metals. The silica hydrogel shows consistently superior adsorption capacity for the trace metal impurities in the oil.
Figure 5. Removal of trace metals from soybean oil.
Application of Silica Hydrogel in Edible Oil Refining
The high adsorption capacity and affinity of silica hydrogels for phospholipids, trace metals and soaps make these synthetic amorphous silicas ideally suited for use in a range of edible oil refining process. Free-flowing silica hydrogel powders can be accurately dispensed and easily dispersed into the oil.
Silica hydrogel does not adsorb color bodies, such as chlorophyll and carotene, from the oil. Therefore, if color removal is required then a combined treatment with silica hydrogel and bleaching adsorbents is necessary. In this case, the order of adsorbent addition is very important. The oil is preferably treated sequentially, first with silica hydrogel and then with bleaching clay. In the first stage, the silica adsorbs the phospholipids, trace metals and soaps. In the second stage sufficient bleaching earth is added to remove the color bodies.
Chemical Refining
Chemical refining of triglyceride oils includes a caustic neutralization step followed by washing[7]. This chemical neutralization, normally using sodium hydroxide, converts free fatty acids to soaps and assists in the hydration of non-hydrateable phospholipids.
After caustic neutralization, the soap and phospholipid contaminants are separated from the oil phase by centrifugation in a continuous process. However, after the primary centrifuge, the oil still contains significant levels of soap and phospholipids. Typically, the soap content may be 300ppm and the phosphorus level may be 10ppm. Consequently, the oil is then washed with water to achieve a final soap level of
Neutralization and washing steps in the chemical refining process are followed by a bleaching step. Bleaching involves the adsorptive removal of color pigments and any residual levels of soap and phosphorus remaining from the caustic neutralization and washing stages. The adsorbent traditionally used is a bleaching clay, which is an acid activated earth based on montmorillonite clay. The surface of the activated clay has a number of Bronsted and Lewis acid sites which are the seat of bleaching activity[2,3].
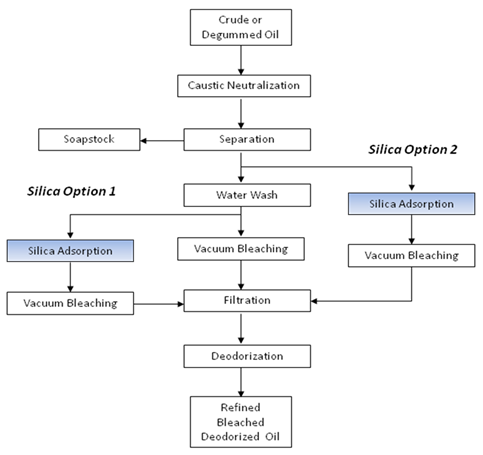
Amorphous silica hydrogel adsorbents can be used in chemical refining in one of two ways, as shown schematically in Figure 6. Silica hydrogel can be used to replace either part of the bleaching earth or to reduce the water washing requirement.
Figure 6. Use of silica hydrogel adsorbents in a typical chemical refining process.
Without modifying the caustic neutralization and washing process, the silica can be added after the final wash stage to remove residual levels of soaps and phospholipids(Silica Option 1). Here the residual level of soap will normally be less than 50ppm and possibly as low as 10ppm. The residual phosphorus level will be less than 10ppm and possibly as low as 3ppm. These contaminants are normally removed in the subsequent bleaching stage by the bleaching earth. However, by using silica hydrogel, the level of bleaching earth required and the total amount of adsorbent used are reduced. Consequently, less waste is produced, oil losses are reduced and filter performance is improved, partly due to the higher permeability of the silica and partly due to a lower solids loading on the filter per ton of oil processed.
Alternatively, wash stages can be eliminated from the caustic refining process and the silica hydrogel can be used to adsorb the soap and residual phospholipids(Silica Option 2). The level of soap at the different wash stages depends on the mixing technology used and the effectiveness of the separators. After the primary centrifuge, without water wash, the soap content of the oil may be anything from 200ppm to 600ppm, typically it would be about 300ppm. By using silica hydrogel as replacement for oil washing, the problem of wash water effluent is eliminated and oil losses are reduced. Furthermore, separation centrifuges are eliminated from the process thus reducing energy and maintenance costs.
Physical Refining
Physical refining can be defined as a refining process which does not include a caustic neutralization step. This process is therefore favored for its simplicity and for environmental benefits, since it eliminates the formation of soapstock and aqueous effluent. However, in the absence of caustic neutralization, the phosphorus content of the oil after degumming must be very low so that it can then be effectively bleached using acceptable levels of adsorbent. In the case of physical refining, all of the free fatty acid removal takes place in the subsequent deodorization stage.
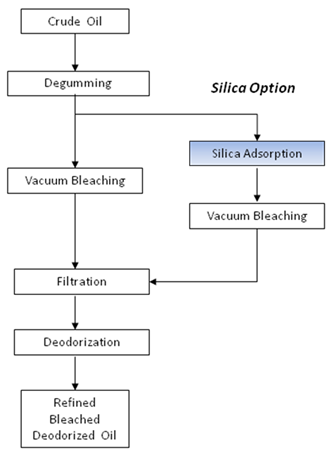
In the physical refining process, the silica hydrogel is used as a partial or even total replacement for the bleaching earth, as shown schematically in Figure 7. Since the physical refining process generally places a greater burden on the adsorbent to remove phosphorus, the excellent phospholipid capacity of silica hydrogel can be of considerable benefit. In some cases, such as the physical refining of super-degummed sunflower oil, where color removal is not a great problem, the bleaching earth can be totally replaced with silica hydrogel. In all cases, the use of silica will reduce overall solids use resulting in less oil loss and better filtration performance.
Figure 7. Use of silica hydrogel adsorbent in a typical physical refining process.
Denickelling
Due to their high affinity for trace metals, silica hydrogel based adsorbents have also found use in the adsorptive removal of residual nickel following hydrogenation of edible oils.
The purpose of the hydrogenation stage is to modify the melting properties of the oil or fat by removing double bonds from the unsaturated fatty acid chains. The hydrogenation takes place at elevated temperatures and pressures in the presence of a nickel catalyst. Following hydrogenation, the nickel catalyst is removed from the oil by filtration. Depending on the filter performance, the use of filter precoat and the oil and catalyst type, the filtered oil may contain residual nickel. Clearly, if the oil or fat is intended for edible purposes any residual nickel must be removed from the oil by further processing. This is sometimes achieved with a post-bleaching step involving adsorptive treatment with bleaching clay.
As with other trace metals, silica hydrogel exhibits excellent capacity for nickel. Silica hydrogel can therefore be used in the post bleaching stage as a partial or, very often, complete replacement for the bleaching earth. Silica hydrogel has approximately 3 to 5 times the capacity of bleaching earth for dissolved nickel in the oil. Therefore its use in denickelling can reduce overall adsorbent loading and solids waste as well as improving filter performance.
Interesterification
As an alternative to hydrogenation, the melting profile of certain types of oils and fats may be modified by a process of interesterification. This process involves the rearrangement of free-fatty acid chains on the triglyceride molecules to produce a distribution of triglycerides with a different melting point.
The interesterification reaction can be catalysed chemically or enzymatically. Chemically catalysed interesterifcation involves the use of sodium methoxide as the catalyst. At the end of the rearrangement reaction, the homogeneous catalyst is destroyed by neutralization, normally with acidified water. This neutralization step also results in the formation of sodium soaps, as sodium hydroxide formed from the hydrolysis of the catalyst reacts with residual free fatty acids. Residual soap levels can be very significant, sometimes in excess of 1000ppm. The residual soap can be removed from the oil by combination of water washing, acidification and post bleaching.
Due to its high affinity for soap molecules, silica hydrogel has found use in the post treatment of interesterified oils and fats. Its greater adsorption capacity for soap compared with bleaching clays is of great significance at these high soap levels, where treatment with clay would require excessive levels of adsorbent. Furthermore, interesterification is normally operated as a batch process and so subsequent washing and separation steps can very time consuming. The use of silica hydrogel allows a reduction in the levels of washing required thereby reducing batch cycle times. In addition, the use of silica reduces the level of waste effluent and associated oil losses.
Oil Processing – Beyond Edible Oils
The affinity of silica hydrogel for polar impurities is applicable in a number of related triglyceride oil and fatty acid processing industries in addition to edible oil refining.
The capacity of silica hydrogel to adsorb trace metals from oils and fats has found application in the oleochemical industry, where silica adsorbents have been used to remove residual catalyst metals after esterification and hydrogenation reactions. Silica hydrogel has been shown to be an effective adsorbent in the removal of tin and zinc catalyst residues following the esterification of fatty acids.
The rapidly expanding global interest in biofuels and other industrial applications for fats and oils has presented new opportunities for silica hydrogel based adsorbents.
Biodiesel is produced by the reaction of a triglyceride oil or fat (edible or non-edible) with methanol to form the fatty acid methyl ester(biodiesel) and glycerin by-product. Apart from the obvious separation from the glycerin, the biodiesel must undergo further treatment before it can be used as a fuel. The biodiesel from the transesterification reaction may contain trace levels of unreacted methanol, monoglycerides, diglycerides and moisture. Also, if a sodium methoxide homogeneous catalyst is used in the biodiesel production then the product will contain sodium soaps. Silica hydrogel can be used to assist in the removal of some of these impurities from the biodiesel. More importantly, silica hydrogel can be used in the pretreatment of the oil or fat feedstock prior to the transesterification reaction.
Availability and cost of feedstock are significant factors in the economics of biodiesel production. Many of the small to medium scale biodiesel producers have used refined bleached deodorized(RBD) oil of edible quality as their feedstock. As the cost of RBD oil increases, these producers have difficulty producing biodiesel profitably, as feedstock accounts for more than 80% of their production cost. Many of the larger-scale biodiesel producers are back integrated into feedstock refining and are capable of handling a wide variety of feedstocks, switching between them to process the lowest cost, widely available feedstock at any time. This presents a significant opportunity for silica hydrogel based adsorbents.
The availability of low cost, high quality feedstock is key to efficient biodiesel production. Contaminants such as soaps and phospholipids will result in higher catalyst consumption, which can be very costly. They will also result in the formation of emulsions which make separation from the glycerin by product difficult and thereby reduce yield. Other impurities in the feedstock, such as trace metals, may have an adverse effect on the biodiesel properties.
Silica hydrogel can be used in the pretreatment of biodiesel feedstocks in much the same way as it is used in edible oil processing. It can be used to adsorb soaps, phospholipids and trace metals from the oil or fat. It very much simplifies a chemical refining process, as no bleaching clay adsorbent is required for color removal from the biodiesel feedstock. Consequently, after neutralization with caustic and separation of most of the soap with a centrifuge, the oil can then simply be treated with silica adsorbent to produce a feedstock suitable for biodiesel production. Alternatively, if the feedstock is physically refined, silica hydrogel can be used to remove phospholipids and trace metals to produce good quality feedstock. Silica hydrogel will also help remove traces of iron from the feedstock, potentially improving the oxidative stability of the biodiesel. Oxidative stability is of growing interest to biodiesel producers and it is now included in the ASTM(United States) and EN(European) specifications for biodiesel. Silica hydrogel will also help in the removal of a range of other polar impurities from animal fat based feedstock.
References
- Mag, T.K. Proceedings of the World Conference of Edible Fats and Oil Processing, Maastricht, 1989, p. 107
- Patterson, H.B.W. Bleaching and Purifying Fats and Oils. Theory and Practice. AOCS Press, 1992.
- Taylor, D.R. Bleaching. p. 285. Bailey’s Industrial Oil and Fat Products, 6th edition, John Wiley & Sons, 2005.
- Iler, R.K. The Chemistry of Silica. Wiley, New York, 1979, pp462-621.
- Welsh, W.A., Bogdanor, J.M. and Toeneboehn, G.J. Proceedings of the World Conference of Edible Fats and Oil Processing, Maastricht, 1989, p189.
- Nock, A. Environmental Issues Facing the Edible Oil industry, SCI Oils & Fats Group, London,1996, p57. Pub PJ Barnes & Associates, Bridgwater, England.
- Hendrix, B. Proceedings of the World Conference of Edible Fats and Oil Processing, Maastricht, 1989, p94.
In This Section
- Marine Oils
- Animal Fats
- Olive Oil
- Palm Oil
- Seed Preparation
- Expanding and Expelling
- Solvent Extraction
- Meal Desolventizing, Toasting, Drying and Cooling
- Introduction to Degumming
- Chemical Degumming
- Enzymatic Degumming
- Alkali Refining
- Optimization of Bleaching Process
- Silica Hydrogel and its Use in Edible Oil Processing
- Deodorization
- Hydrogenation Mechanism
- Chemical Interesterification
- Enzymatic Interesterification
- Solvent Fractionation
- Dry Fractionation
- Hydrogenation in Practice