1. Introduction
The benefit of using enzymes as auxiliary agents in degumming vegetable oils is that it reduces total oil loss in the degumming process. Obviously, reduction of oil losses must pay for the enzyme cost, plus additional energy (power and heat) and additional capital costs required in the plant when compared to conventional processes (water degumming or acid degumming, caustic refining). It is the opinion of the authors that the apparent slow advance of enzyme degumming is, in great part, due to the lack of reliable, independent data on total process performance, which allows for the calculation of process economics.
The current stage of the industry facilitates adoption of enzyme processes. Vegetable oil prices are currently high, generating a spread over meal. As most degumming plants are not producing food- or feed-grade lecithin, the gums are typically returned to the meal, and the value for the additional oil yields is relatively straightforward to calculate. The level of automation currently existing in crushing and refining plants is also favorable for controlling the process and allows for optimal control of enzyme performance. The major variables of the value equation are the enzyme cost, which can and should be provided by enzyme suppliers on a case-by-case basis, and a forecast of enzyme efficiency, which depends on published data.
Separate articles on this website provide an Introduction to Degumming and describe Chemical Degumming.
2. Endogenous Phospholipids Found in Crude Soybean Oil
After the miscella distillation, crude soybean oil contains many impurities. Phospholipids are the highest impurity [1]. The same can be said for crude rapeseed/canola oil.
The composition of phospholipids has historically been determined by either thin layer chromatography (TLC) or column chromatography [2]. Recently, phosphorus-31 (31P) NMR was developed as an alternative and accurate way to measure the amounts of the different phospholipids in vegetable oil [3]. None of these techniques are however available on site, and most often not even in analytical research or commercial labs that support the oils and fats industry. As an industry standard, plants usually measure the elemental phosphorus (P) in the oil, as an indication of the presence of phospholipids. The adaptation of inductively coupled plasma (ICP) has allowed for fast screening of the phosphorus, as well as other metal contaminants, in oil during the various process stages and the final refined, bleached, deodorized (RBD) oil quality.
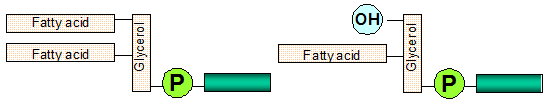
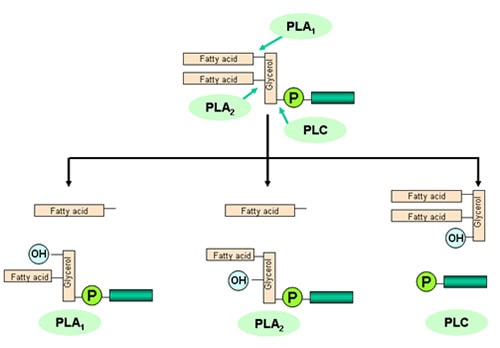
Elemental phosphorus measurements do not indicate the quantity of each of the common phospholipid species found in the oil, namely phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI) and phosphatidic acid (PA). Nor do they identify other compounds containing phosphorus, including degraded forms of phospholipids (such as lyso-phospholipids; Fig. 1).
Figure 1. Representation of molecular structures of phospholipids (left) and lyso-phospholipids (right); the nature of the ester group attached to the phosphorus determines its polarity (courtesy of Verenium).
The affinity for water of the ester group determines the overall affinity for water of the phospholipid: the higher the water affinity, the higher the emulsification power. The relative affinity of phospholipids for water is usually called “hydratability.” Those phospholipids responsible for higher oil losses due to oil emulsion, i.e. PC and PI, are usually designated as hydratable phospholipids (HP), while PA and PE salts of calcium (Ca), magnesium (Mg) and iron (Fe) are usually called nonhydratable phospholipids (NHP).
Sen Gupta [4] published data on the relative rate of hydration of the different phospholipids found in vegetable oils. Sen Gupta’s results indicate that hydration of PI takes approximately twice as much time of that of PC (Table 1). From the same data, hydration of the acid form of PA takes approximately ten times longer than that of PC. The experiments demonstrate that the hydration time for the calcium salts of PA and PE is more than 150 times longer than that of PC, such that these salt forms of phospholipids are indeed “nonhydratable” under usual degumming plant conditions.
| Table 1. Relative rates of phospholipid hydration – adapted from [1] after [4] | |
| Phospholipid | Relative rate of hydration |
| Phosphatidylcholine | 100 |
| Phosphatidylinositol | 44 |
| Phosphatidylinositol (calcium salt) | 24 |
| Phosphatidylethanolamine | 16 |
| Phosphatidic acid | 8.5 |
| Phosphatidylethanolamine (calcium salt) | 0.9 |
| Phosphatidic acid (calcium salt) | 0.6 |
The authors find the table illustrative of the mechanism of phospholipids removal by various degumming processes. In water degumming, the Ca, Mg and Fe salt forms of the PA and PE are not hydrated, under plant conditions, therefore remaining in the oil. In the case of chemical degumming, when correctly performed, the metals mentioned can be removed from the phospholipids, and the acid forms of PA and PE can be removed in the gums, producing oil with very low phosphorus levels at the expense of losses higher than that of water degumming. In the case of enzyme degumming, the final level of phosphorus in the oil will depend both on the efficiency of the removal of metals from the PA and PE, and on the specificity of the enzyme and the conversion of the phospholipids in its reacted forms, either more polar (such as lysophospholipids and phosphoro-esters) or very nonpolar (fatty acids and diglycerides). In the end, each of the different processes generates a different oil quality, fatty matter loss and cost of processing. This article covers both the different qualities of the oils produced and the fatty matter loss that can be expected when oils with high and very high phospholipid content are degummed using different degumming methods.
The relative concentrations of different phospholipid species are not constant in crude oils, and a large variation in the relative amounts of PC and PA is observed in different production facilities. The amount of each kind of phospholipid in the crude oil depends not only on the quality of the beans but also on the seed preparation and oil extraction processes [5]. PA accounts for 7 to 20% of the phospholipids found in crude soybean oil – and 6 to 60% in crude rapeseed oil – this range apparently caused by the use of expeller-presses and/or solvent extraction, which yield different phospholipid composition (data from the authors). In the case of crude-degummed oils (oils that were previously water degummed), the PA (in its salt form) usually corresponds to 60 to 100% of the phospholipids. The calcium and magnesium salt forms of PA are particularly difficult to remove from the oil because their very low polarity makes them highly oil-soluble.
The phospholipid distribution observed in oil extracted from an average quality soybean processed in a standard crushing plant using expanders for 100% of the plant capacity is shown in Table 2, as well as crude rapeseed oil – in this case, variation can also be explained by the amount of pressed versus solvent-extracted oils.
| Table 2. Soybean data from the authors (phosphorus by ICP; phospholipids by 31P-NMR, analysis conducted by Verenium); rapeseed data from several authors and sources | |||
| Phosphorus and phospholipid profile of crude oils | Soybean | Rapeseed | |
| Phosphorus content (ppm) | 850-1200 | 200-900 | |
| Phospholipid content (%) | 2.0-2.9 | 0.5-2.3 | |
| Relative phospholipid distribution (%) |
Phosphatidylcholine | 47 | 27 |
| Phosphatidylinositol | 24 | 17 | |
| Phosphatidylethanolamine | 20 | 17 | |
| Phosphatidic acid | 9 | 39 | |
3. Oil Yield Considerations for Water Degumming
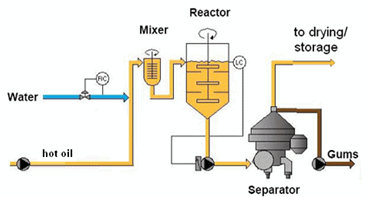
Water degumming is still a common process for removing phospholipids from crude oil (Fig. 2). In this process, warm water is added to the crude oil at 80-85°C and the mixture is agitated slowly for approximately 20 minutes, a process usually referred to as “hydration”. The water dosage used is usually based on the expected amount of phospholipids in the crude oil. The “hydratable” phospholipids agglomerate at the interface of the oil and water, capturing some nonhydratable phospholipids with them. Oil is also trapped by the phospholipids, forming an emulsion, referred to as “gums” or “wet gums”.
Figure 2. Simplified scheme of water degumming process (courtesy Alfa Laval).
Usually the actual phospholipid content of the crude oil is unknown in the production facility and the phosphorus content of the oil is used as a proxy. The calculated conversion of phosphorus to phospholipid content is normally made using an assumed ratio of the atomic weight of 31 for phosphorus (P) and the molecular weight of phospholipids (PL) all of which contain one phosphorus atom. Based on the estimated molecular weight of each of the phospholipids in oil, the average P to PL ratio would be around 25 for a plant running with expander on 100% of capacity (Table 3).
| Table 3. Molecular weights of phospholipids calculated by the authors assuming C18:1 as the acyl group and structures proposed by [6] | ||
| Common phospholipids found in vegetable oils | Estimated molecular weight | Phosphorus to phospholipid conversion factor (P/PL) |
| Phosphatidylcholine | 784 | 25 |
| Phosphatidylinositol | 861 | 28 |
| Phosphatidylethanolamine | 742 | 24 |
| Phosphatidic acid | 699 | 23 |
A discrepancy has been observed between the measured phosphorus content of crude oils and the phospholipid content measured by 31P NMR (Table 4). The authors therefore conclude that other phosphorus-bearing compounds are present in the crude oil, which can account for 10 to 30% of the total elemental phosphorus determined by ICP. The ratio of P to PL, however, appears to be fairly constant in a given production facility, indicating that these other phosphorus-containing impurities may be more (or less) extracted depending on the preparation and extraction conditions used. It is highly recommended that every site be evaluated for its real (average) P to PL, and that a ratio of P bound to PL and total P is determined allowing for better estimation of PL content in crude oil on a daily basis.
| Table 4. Data from the authors, corresponding to a single industrial plant (P by ICP; PL by 31P-NMR; analysis conducted by Verenium) | |||||
| Sample | Total phospholipids (%) | Total lyso-phospholipids (%) | Total phosphorus (ppm) | Phosphorus bound to phospholipids (ppm) | P in PL/P total |
| Crude oil | 2.0 | 0.1 | 1043 | 824 | 0.79 |
There is also some variation in the absolute PC content of the crude oil and in the PC content as a proportion of total PL in the oil. Therefore, the emulsification losses will always vary in industrial scale applications. Regardless of process control/optimization attempts, variable yields in water degumming are unavoidable even under tight control of process parameters (e.g. water dosage, temperature of oil and water, hydration time, and centrifuge adjustment parameters).
The two standard measurements of process efficiency in water degumming are the P to Ca and Mg ratio, which indicates the removal of all hydratable phospholipids from the oil, and the determination of the acetone-insoluble (AI) content of the gums, which indicates the amount of oil lost. Phospholipids, lysophospholipids, glycolipids and other polar compounds are insoluble in acetone, while triglycerides, diglycerides and fatty acids are soluble in acetone. Therefore, the AI analysis enables a ratio between oil and impurities present in the gums to be determined, with the phospholipid content corresponding to the major part of the acetone-insoluble fraction.
The residual phosphorus levels found in the water degummed oil average between 80 and 130 ppm, and correlate very well with the remaining metal (Ca, Mg, Fe) content of the oil because the metals are bound to the phospholipids, particularly PA, forming nonhydratable salts.
Formulae have been proposed to estimate the oil and fatty matter losses in degumming, using the acetone-insoluble analysis of the gums [7]. Due to their simplicity, these formulae are adequate to be used as a fast approximation of plant performance, but they lack accuracy because they do not account for the real cause for the losses, i.e. the amount of phospholipids present, particularly PC, which is not determined at plant level.
% oil lost in gums = ΔP x K / 10000 x ((100-AI)/AI)
% fatty matter lost in gums = ΔP x K / 10000 x (1+((100-AI)/AI))
where –
- AI indicates the % of acetone insoluble in gums (%)
- ΔP is the difference between the phosphorus (ppm) in the crude and degummed oil
- K is the P to PL ratio, usually assumed as around 30, which represents the P/PL ratio of 25 plus a compensation for other impurities insoluble in acetone.
The acetone-insoluble content of gums obtained via water degumming can vary usually from 62 to 70% – indicating roughly 30 to 38% of oil, fatty acids and lipids other than phospholipids in the gums. With no knowledge of the real amount of phospholipids in the gums, and particularly the amount of PC, AI% is only an indirect measurement of the polar and nonpolar compounds in the gums, not actually a mass balance of losses. Furthermore, AI% varies when process conditions are intentionally or unintentionally changed. This variation is caused by changes in concentration of polar contaminants, such as phospholipids, and their composition. The authors therefore assume that the AI% is only an approximate indication of process efficiency, but insufficient to guide process optimization studies.
4. Oil Yield Considerations for Chemical Degumming Processes
It is known that oil submitted to a thorough removal of phosphorus is suitable for physical refining. For physical refining, the recommended maximum phosphorus level in oil before bleaching is 3 ppm [8]. Given optimal process conditions (acid, acid retention time, caustic), even the so called “nonhydratable” phospholipids can be removed via hydration. The low phosphorus levels in chemical degumming can only be obtained at the cost of losses higher than with water degumming. In addition to the higher amounts of phospholipids removed from the oil, the gums processed by acid degumming have usually lower AI value, 50-60% [8], indicating 40 to 50% oil, fatty acids and lipid material other than phospholipids in the gums.
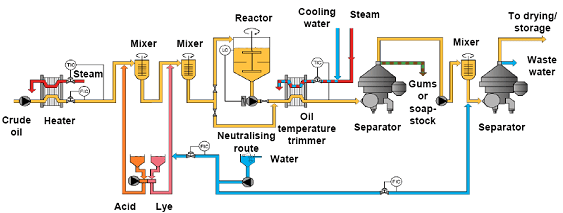
In some forms of chemical degumming (Fig. 3), caustic is added after the acid treatment, increasing the pH [1,7,8]. This allows for the reduction of the viscosity of the acid gums, and also the reduction of the residual phosphorus levels in the oil as the caustic forms soaps having great emulsification properties, improving the removal of the so-called NHP. With the use of these techniques, phosphorus levels lower than 20 ppm can be achieved in the degummed oil, particularly after a water-wash.
Figure 3. Simplified scheme of acid degumming followed by caustic treatment and washing (courtesy of Alfa Laval).
The practical limit for oil losses in the chemical degumming processes is composed of the amount of phospholipids to be removed, and the oil they emulsify. The only way to reduce fatty matter loss even further would be through changes in the emulsifying nature of the phospholipids themselves. This is what enzymes can do.
5. Degumming with Enzymes – How the Process Came to Be
Several authors have published data on plant-scale performance of enzyme assisted degumming processes. The summary below is not considered to be a thorough list of publications on the topic, but the ones that can be considered relevant to the practices currently in use in the industry.
Röhm GmbH (later Lurgi) developed the first enzymatic process for degumming edible oils, the EnzyMax® process, patented by Aalrust et al. [9]. The process initially used a phospholipase A2 (PLA2) from porcine pancreas produced by Röhm. The original process described by Aalrust et al. dissolved the enzyme in water with sodium citrate and sodium dodecyl sulfate, which is a very strong emulsifier. The enzyme/water mixture was then added to oil that was previously heated to 50 to 75°C – indeed the porcine enzyme is quite heat tolerant. An emulsion was formed and the enzyme was allowed to react with the phospholipids from 3 to 4 hours before centrifugation which resulted in residual phosphorus in the oil of 3 ppm. The patent authors ruled out the use of crude (high phosphorus) oils, focusing on using oil rich in NHP and less than 250 ppm of phosphorus – assuming higher value for lecithin. They also didn’t see value in the use of phospholipases C or D –though none of these were commercially available at the time anyway. Limited amounts of the enzyme were available, the enzyme was expensive and a “recirculation” of the gums was proposed – which is not clear as having ever being proven to be economical. Aalrust et al. did not publish compositional data on the gums obtained after the reaction and separation in the industrial scale application, therefore not allowing for an economic assessment of this solution.
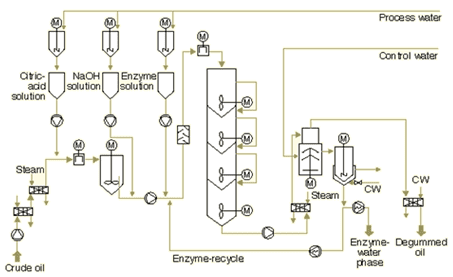
Dahlke [10,11] provided more detail on the EnzyMax® process (Fig. 4). They reinforce the idea that the process was designed to only remove nonhydratable phospholipids from the oil and that only a PLA2 would be able to economically perform a full degumming. According to Dahlke, the lyso-phosphatide resulting from the enzyme reaction is insoluble in oil, and therefore removed in the water phase, and the free fatty acid formed remains in the oil.
Figure 4. EnzyMax® process according to Dahlke [10].
He also describes the process phases for the enzyme degumming:
- Adjusting the optimum temperature of the oil for the enzyme reaction;
- Adding citric acid and retention of the oil with acid for a period of time;
- Adding caustic soda to buffer the water phase to the expected optimal pH for the enzyme to be used;
- Adding enzyme and water to the oil, via mixers (static and mechanical) – gums were partially re-circulated here when using the porcine enzyme;
- Reacting the NHP in the reaction tank (up to six hours);
- Heating the oil to the optimal temperature for separation in the centrifuge; and
- Separating lyso-phosphatides from the oil in the centrifuge
Both Aalrust and Dahlke claim the importance of fine dispersion of the buffered water + enzyme + oil mixture, but no details are provided regarding the nature of such mixers. The original patent and subsequent publication mention only that the enzyme dispersion can be achieved with “conventional equipment”, meaning mixers used at the time in oil processing, which unfortunately was proven later not to be the case in a robust, industrial-scale application. Details of the reaction tank are not provided, except that it consists of a 6 compartment tank, reducing the possibility of short circuiting the reaction time, and the graphics indicate the need for agitation in each compartment. Both soybean (65 ppm P before reaction) and rapeseed (100 to 200 ppm before reaction) oils are claimed by Dahlke to be successfully processed in such manner in a 500 t/d industrial plant, with residual phosphorus levels below 10 ppm in the oil after centrifugation of the reacted oil. The enzyme recycle is not detailed by the author.
Clausen et al. [12,13] describe a new PLA1, Lecitase® Novo, obtained from Fusarium oxysporum. This new enzyme, obtained by fermentation, opened the possibility for higher volumes and lower costs for the enzyme. This allowed to the enzyme not to be recycled, allowing for optimal process control.
Münch [14] reports results with both Lecitase® 10 L with gums (and enzyme) in recirculation, operated for a period of 5 years, and the Lecitase® Novo, without enzyme recirculation, for a period of 1 year. Both enzymes were claimed to have delivered degummed rapeseed oil with less than 10 ppm of phosphorus. Initial phosphorus in the oil was 100-280 ppm. Some data are presented on Lecitase® Novo being able to allow for total conversion of phospholipids into their lyso forms after 3 hours of reaction. No data on oil yields are provided.
Yang et al. [15] report an optimization experiment for the use of a new enzyme, Lecitase® Ultra, obtained from Thermomyces lanuginosea Fusarium oxysporum. Rapeseed oil was used, apparently with a process layout very similar to the one proposed by Dahlke. The process was optimized for reduced phosphorus in the degummed oil, not optimizing the oil yield. In the plant scale trial, oil with 120.5 ppm of phosphorus (apparently previously water degummed) was submitted to the following reaction conditions:
- Oil flow, 17 t/h.
- Citric acid treatment – citric concentration 45%, citric flow of 20 L/h, citric retention time of 30 minutes.
- Caustic solution – dosage and concentration not mentioned, claimed to be enough to control pH of water phase between 4.6-5.1.
- Enzyme solution – 8.8 L/h, claimed to be equivalent to 40 ppm of oil flow.
- Reaction – temperature of 48 ± 2°C, 6 hours reaction time.
The reacted oil was later submitted to bleaching and physical refining. The author did not report oil content in the gums, or the reaction profile of the phospholipids. Results on oil yields were not included in the paper.
Chakrabarti et al. [16] disclosed the use of Lecitase® Novo for rice bran oil, with acid pretreatment and caustic buffer and enzyme reaction of up to 110 min. The oil obtained after centrifugation was bleached with 2 to 4% bleaching clay and zero to 1% activated carbon. The oil was then de-waxed to achieve oil with a phosphorus content of 1 to 3 ppm. The authors did not report phosphorus after the centrifugation, or disclose the breakdown of phospholipids in the gums, or additional yield obtained in the physical refining plant compared with conventional processes. The somewhat high phosphorus levels after bleaching and the claimed oil content in the gums of 30 to 40% indicate that the enzyme reaction did not go to completion.
Dayton et al. [17] describe the addition of acids to lower the pH of the water phase after the enzyme reaction to avoid deposits of calcium and magnesium citrate salts in the downstream processing equipment, notably heat exchanger and centrifuge discs. Despite not changing the performance of the enzyme or oil yields, the technology allows for the enzyme process using citric acid to run continuously, without interruptions for cleaning.
Gramatikova et al. [18] describe the use of a new phospholipase with PLC activity, Purifine™, produced by Verenium. The enzyme is reported to be able to hydrolyze the phosphorylcholine and phosphorylethanolamine bonds (that occur in up to 70% of phospholipids in crude soybean oil) in a two-hour reaction, using water without the use of a pH buffer. The authors did not offer plant scale data on oil yields.
Dayton and Galhardo [19] describe the use of a combination of PLC and PLA enzymes for the complete reaction of phospholipids in a degumming process. A synergy between the two different types of enzymes was discovered, even when reaction conditions are not optimal for both. Reaction time was greatly reduced to 30 min – 1 hour. In the patent application, the inventors did not offer plant scale data on oil yields.
6. Use of Phospholipases to Reduce the Emulsification Properties of the Phospholipids
One way to reduce the emulsification properties of the phospholipids is to cleave or cut their polar and nonpolar parts from one another. Enzymes are an effective way to achieve this reaction. They are natural, selective catalysts, reacting at moderate temperature and pH. Compared with chemical processes, enzyme-catalyzed reactions are very selective, greatly reducing or eliminating the formation of undesired by-products. The enzymes of interest which are active on phospholipids are called phospholipases.
Literature reports several kinds of phospholipases (Fig. 5). The enzymes able to remove fatty acids from the phospholipids are conventionally named hospholipase A (PLA) (1 or 2, according to the position of the fatty acid removed from the glycerol backbone) and phospholipase B (PLB) (able to remove the remaining fatty acid from a lysophospholipid). Phospholipase C (PLC) is able to remove the “phosphoro-ester” group and generate a diglyceride for each phospholipid molecule reacted. Phospholipase D (PLD) is present in seeds and is responsible for the destruction of PC and formation of PA during storage and processing.
Figure 5. Phospholipases of interest for vegetable oil processing and reaction products (courtesy Verenium).
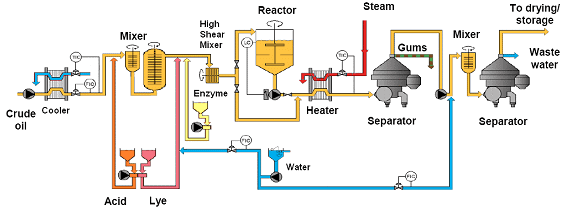
The use of enzymes for degumming is usually referred to as “enzymatic degumming,” and process conditions are designed to optimize the activity of the enzyme used (Fig. 6). This technique is useful for both crude and crude degummed oils (oils that were previously water degummed).
Figure 6. Simplified scheme of enzymatic degumming process (courtesy Alfa Laval).
The use of chemicals (acid and caustic) has a dual role – changing hydratability of some phospholipids, but also optimizing the enzyme performance regarding the pH of the oil-water mixture. If only hydratable phospholipids are targeted for the enzymatic reaction, the use of additional chemicals (acid and caustic) may be eliminated. The crude oil has to be cooled to the temperature that is optimal for the enzyme performance (40° to 70°C for commercially available enzymes). This also has the benefit of allowing the formation of a stable emulsion required for reaction and preventing any unwanted saponification from the addition of caustic soda in the next step.
Caustic soda is then necessary to buffer the pH of the water phase, in order to allow the enzyme the optimum pH condition to for its reaction. Water is added to the cool oil, in a proportion of up to two times the amount of phospholipids present, and the enzyme is dosed according to the recommendation of the supplier (usually 30 to 200 ppm calculated as w/w% of the oil flow).
A very high shear mixer, such as the IKA Dispax, is used to produce a stable emulsion with the enzyme in the water phase and the hydratable phospholipids deposited at the oil-water interface. The emulsion is maintained by agitation until all the targeted phospholipids are reacted (since activity varies for each commercially available enzyme). Once the reaction is complete, phospholipids have been converted into their reaction products (lysophospholipids and fatty acids, or DAG and phosphorylesters). The oil/water mixture is then heated up to 75-85°C and separated using a self-cleaning centrifuge.
Each commercially available enzyme (Table 5) provides different economic benefits due to the differences in selectivities and the price per dosage.
| Table 5. Commercially available phospholipases for enzyme degumming | |||
| Supplier | Brand | Activity | Organism of origin |
| AB Enzymes | Rohalase®MPL | PLA2 | Trichoderma reesei [20] |
| Danisco | Lysomax® | PLA2 | Streptomyces violaceoruber [21] |
| DSM | Gumzyme® | PLA2 | Aspergillus niger [22] |
| Novozymes | Lecitase®10L | PLA2 | Porcine pancreas [21] |
| Novozymes | Lecitase® Novo | PLA1 | Fusarium oxysporum [15] |
| Novozymes | Lecitase® Ultra | PLA1 | Thermomyces lanuginosa / Fusarium oxysporum [15] |
| Verenium | Purifine™ PLC | PLC | Pichia pastoris [23] |
In this web article results of usage of two enzymes in crude soybean oil are presented. The enzymes used were the Lecitase® Ultra PLA1 from Novozymes, and the Purifine® PLC from Verenium.
7. Use of Lecitase® Ultra PLA from Novozymes on an Industrial Scale
Lecitase® Ultra PLA from Novozymes has an optimal reaction pH of around 4.5 in the water within the oil matrix. This enzyme reacts with all phospholipids (PC, PI, PE and PA), but it is the experience of the authors that the required reaction times greatly vary from one phospholipid to another, Ca, Mg, and Fe salts of PA being the slowest to react, taking from 4 to 6 hours.
It should be noted that operating at pH of 4.5 or lower requires plant construction in stainless steel. It has also been noted that calcium salt deposits (apparently calcium citrate) form in the oil heater and on the centrifuge disks, but this problem is eliminated when the pH is reduced further [16].
The oil obtained with Lecitase® Ultra has very low residual phosphorus (<10 ppm, usually <5 ppm), without the need of a water wash. The degummed oil has excellent quality for physical refining. An FFA balance, done by HPLC, indicated that the FFA content in the oil is increased, as expected with the use of a PLA, and it has been observed that approximately half of the FFA generated remains in the oil while the rest is lost in the reacted gums.
The expected FFA increase in the treated oil can be calculated from the original amount of phospholipids present in the crude multiplied by the ratio of molecular weights of FFA and phospholipids, approximately 282/750, and the assumption that half of the fatty acids stay in the oil. Thus, an oil with 2% phospholipids would see an increase in FFA content of approximately 0.38% (2% × 282/750 × 1/2).
Phospholipids are almost totally converted into lysophospholipids by PLA, and the presence of PA and PE in the gums, and the PA in the degummed oil are indications of incomplete reaction. Reacted gums obtained with the Lecitase® Ultra have reduced oil content (around 15%). Higher oil content in the gums may indicate partial reaction or suboptimal centrifuge performance, as the centrifuge has to be adjusted to the new viscosity and density of lower amounts of gums. Part of the “oil” in the gums arises from (half of) the fatty acids generated in the reaction. The reduction of the absolute amount of oil in the gums, the lower molecular weight of the lysophospholipids produced and the acetone-soluble fatty acids found in the reacted gums result in an AI% in the dried gums (60-65%) that is comparable with that of water degumming (62-70%). However, it is of paramount importance to note that the gum sample is visibly distinct: much less viscous, with a pale yellow color, and the volume of the reacted gums is greatly reduced.
8. Use of Purifine® PLC from Verenium on an Industrial Scale
Purifine® PLC from Verenium has an optimum pH range of 5.5-8, therefore the use of citric acid and caustic soda as a pH buffer is optional. This enzyme is selective only for the PC and PE, with reaction time of a maximum of 2 hours. It is noted that, if the pH buffer is not used, the plant can be built in carbon steel.
The oil obtained with Purifine® PLC has a phosphorus content comparable with water degummed oil, with P levels of 50 to 100 ppm depending on the amount of PA and metals present in the crude. The free fatty acid content of the oil does not increase with the enzyme reaction, because the hydrophobic reaction product is a diglyceride (DAG). The amount of DAG likely to be generated can be calculated using the original PC+PE content of the oil multiplied by the molecular weights ratio 605/750, and an observed reaction efficiency of ~85% (tentatively explained by the complete reaction of the PC, but only partial reaction of the PE, due to the relative rate of its hydration). In practice, all the DAG formed remains in the degummed oil. As an example, a crude oil with 2% of phospholipids is expected to have approximately 1.3% PC+PE, allowing a maximum yield of 0.89% DAG (1.3% × 605/750 × 0.85).
The heavy phase discharged from the centrifuge will contain phosphorylesters of choline and ethanolamine, as well as all the PI (unreacted) initially present in the crude oil, some unreacted PE, and oil.
The nonhydratable phospholipids (NHP), mainly in the form of metal salts of PA and PE, remain in the oil and do not cause oil losses in the degumming stage. The remaining emulsifying property of the gums is due to the PI, which has been empirically observed to be reduced when compared with gums also containing unreacted PC. As a result, the reacted gums obtained with the use of Purifine® PLC contain a reduced amount of oil (10-20%, wet basis). Higher oil content in the gums may indicate partial enzyme reaction or suboptimal centrifuge performance, as the centrifuge has to be adjusted to the new viscosity and density of lesser amounts of gums.
The reacted gum amount is approximately one-third of that obtained when using water degumming, because the majority of the mass of the phospholipids is released as DAG and the remaining phospholipids have lower emulsification properties. It is important to note that the AI% of the reacted gums when using Purifine® PLC (70-75%) is somewhat higher than when using water degumming, and the gums present a very distinct, liquid appearance.
When using acid treatment prior to the reaction with Purifine® PLC, the degummed oil obtained may have a phosphorus level of less than 10 ppm after water wash, and even lower if the crude oil has a low PA content. The oil losses to the reacted gums are higher than when not using the pH buffer, as all the PA and PI are hydrated and removed without being hydrolyzed by the enzyme. However, there is an improvement in the reaction of PE verifying the relative rates of hydration proposed by Sen Gupta [4]. The AI% of the gums is reduced (65-70%), and the neutral oil in the gums reaches >20%. The water wash used to reduce the phosphorus levels in the degummed oil generates additional oil losses (0.2 to 0.3%), due to residual phospholipids and emulsified oil, but the advantage of this process is the possibility to substitute caustic refining as way to prepare the oil for bleaching and physical refining. Silica filtration can replace the water wash.
9. Use of a Combination of Lecitase® Ultra PLA from Novozymes and Purifine® PLC from Verenium on an Industrial Scale
The combination of PLA and PLC enzymes allows the ability to react all of the phospholipids present in crude oil while still maximizing the yield gain from PLC for physical refining of oils, independent of the level of nonhydratable phospholipids present in the feed material. The additional benefit discovered was the synergistic effect of the two enzymes in combination. The presence of PLC allows for a significant increase in the reaction rate of either enzyme alone, regardless of the concentration of the single enzyme. It was found that hydrolysis of all of the phospholipids was completed in as little as 30 minutes [18]. The reaction conditions were the same as PLA alone, except both enzymes were added at the same time and allowed to react. The oil present in the gums was less than 10%, and AI of the gums was 70-75%.
10. Measuring the Reaction Efficiency
The enzyme degumming process greatly changes the physical properties of the resulting gums when compared with conventional degumming. The hydrolyzed gum is less viscous, denser, with a liquid appearance, as it holds less emulsified oil. The hydrolyzed gum is very soluble in warm water.
There are three ways to design a process comparison of enzymatic degumming routes and conventional degumming of crude oils:
- Analytical measurement of the reaction products
This is essential during plant startup. When using Purifine® PLC, the DAG generated is measured by HPLC and the phosphorylesters (and phospholipids originally present in the crude) are measured by 31P-NMR. When using Lecitase® Ultra PLA, the free fatty acids are measured in the plant and the lysophospholipids by 31P-NMR. The 31P-NMR is only performed in a few specialized labs, but unequivocally reflects the enzyme performance. It is strongly recommended that the enzyme suppliers are involved in such investigation in order to help define reference methods, recommended labs and frequency/duration of sampling. - Mass balance using mass flow meters in crude oil and reacted gum tank
This was found to be the easiest daily method for process optimization. In conjunction with measurement of dry matter in the reacted gums, mass flow meters are able to provide a continuous inventory of yields/losses. This is an indirect way of demonstrating the enzyme performance for the reduction of the total mass of gums obtained. - Inventory loss
Less accurate, but over time the additional oil yields should be reflected in the fatty matter balance in the crushing/refining plants.
11. Yield Gains for Enzyme Degumming – Case Studies
Galhardo et al. [24,25] published results on oil yields (Table 6) using different enzymes, using crude soybean oil with 720 and 1000 ppm of phosphorus, as this is representative of crude soybean oil quality worldwide.
| Table 6. Data from authors; modified degumming with Lecitase® Ultra PLA according to [17]; modified degumming with combination of Lecitase® Ultra and Purifine® according to [19]; acid degumming (*) with washing, no enzyme was obtained through theoretical model | |||||||
| Crude oil with 720 ppm P, 1.8% PL, 0.4% FFA and 0.3% DAG (as % of crude oil) | Reaction pH | Reaction/ hydration time (h) | Final P degummed oil (ppm) | Final PL degummed oil (%) | FFA degummed oil (%) | DAG degummed oil (%) | Gums, dry basis |
| Water degumming, no enzyme | Neutral | 0.5 | 120 | 0.29% | 0.3% | 0.3% | 2.7% |
| Water degumming assisted with Purifine® PLC enzyme | Neutral | 2 | 120 | 0.29% | 0.4% | 1.1% | .9% |
| Acid degumming with washing, no enzyme(*) | Acid or caustic | 1 to 2 | <20 | 0.02% | 0.3% | 0.3% | 3.6% |
| Lecitase® Ultra PLA degumming, no washing | Acid (pH 4.5) | 4 to 6 | <10 | 0.02% | 0.7% | 0.3% | 1.9% |
| Combination of Lecitase® Ultra and Purifine® degumming, no washing | Acid (pH 4.5-7.0) | 2 | <10 | 0.02% | 0.6% | 1.2% | 1.2% |
|
|
|||||||
| Crude oil with 1000 ppm P, 2.5% PL, 0.4% FFA and 0.3% DAG | Reaction pH | Reaction/ hydration time (h) | Final P degummed oil (ppm) | Final PL degummed oil (%) | FFA degummed oil (%) | DAG degummed oil (%) | Gums, dry basis |
| Water degumming, no enzymes used | Neutral | 0.5 | 120 | 0.29% | 0.4% | 0.3% | 3.7% |
| Water degumming assisted with Purifine® PLC enzyme | Neutral | 2 | 65 | 0.16% | 0.4% | 1.3% | 1.5% |
| Acid Degumming with washing, no enzyme (*) | Acid (pH 4.5-7.0) | 1 to 2 | 10 | 0.02% | 0.4% | 0.3% | 4.9% |
| Lecitase® Ultra PLA degumming, no washing | Acid (pH 4.5) | 6 to 8 | 10 | 0.02% | 0.8% | 0.3% | 2.3% |
| Modified degumming with Purifine® PLC with washing | Acid (pH 5.5-7.0) | 2 | 10 | 0.02% | 0.4% | 1.4% | 2.2% |
| Combination of Lecitase® Ultra and Purifine® degumming, no washing | Acid (pH 4.5-7.0) | 2 | 10 | 0.02% | 0.9% | 0.9% | 1.1% |
In one set of industrial trials, water degumming assisted with Purifine® PLC enzyme – Purifine® PLC was used in isolation, without the pH buffer (no acid or caustic soda), replacing water degumming. In another set of plant scale experiments, oil with approximately the same amounts of phospholipids were processed in order to remove the maximum amount of phosphorus (phospholipids), in three different ways, as below:
- Lecitase® Ultra PLA degumming, no washing – citric acid and caustic soda as suggested by the supplier followed by Lecitase® Ultra PLA;
- Modified degumming with Purifine® PLC with washing – citric and caustic soda as suggested by the supplier with Purifine® PLC;
- Combination of Lecitase® Ultra and Purifine® degumming, no washing – citric acid and caustic soda followed by Lecitase® and Purifine™
Oil losses were measured in each process alternative (except for acid degumming), using scale tank and mass flow meter for the gums/reacted gums, and confirmed by analytical measurements in the reacted gums. Oil losses for acid degumming were calculated as equivalent to generate gums with AI of 50%.
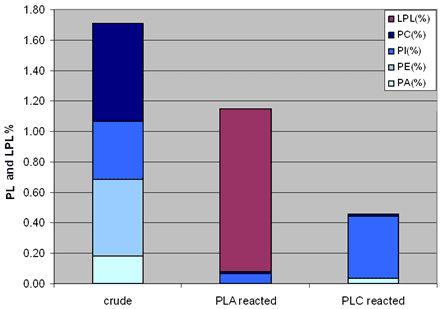
A major conclusion from the tests performed is that the oil losses during degumming with enzymes are not proportional to the amount of phospholipids in the crude – and in fact, losses can be lower than the initial amounts of phospholipids present in the crude, particularly when using a PLC enzyme. This is not intuitive – oils processed by conventional degumming give rise to losses that are always multiples of the amounts of phospholipids. A deeper look into the phospholipid profile (Fig. 7) after the reaction and before the centrifugation illustrates the changes in the oil matrix that allow for the reduced oil losses. In fact, using enzymes allows for a great reduction of the mass of phospholipids. This, added to the lower emulsification properties of by-products, is the basis for the yield gains observed.
Figure 7. Phospholipid (PC, PI, PE and PA) and lysophospholipid profile by 31P-NMR in crude soybean oil submitted to degumming using PLA or PLC enzymes (data from the authors).
References
- Segers, J.C. Degumming – Theory and practice. In: Edible Fats and Oils Processing: Basic Principles and Modern Practices, pp. 88-93 (D.R. Erickson (ed.), American Oil Chemists’ Society, Champaign, IL) (1990).
- Racicot, L.D. and Handel, A.P. Degumming of soybean oil: Quantitative analysis of phospholipids in crude and degummed oil. J. Am. Oil Chem. Soc., 60, 1098-1101 (1983).
- Domaille, P., Huang, H., Marks, S., O’Donoghue, E., Deciu, C., Walsh, D. and Barton, N.R. Analytical monitoring of phospholipase-C mediated vegetable oil degumming. Paper presented at the 99th AOCS Annual Meeting & Expo, Seattle, (2008).
- Sen Gupta, A.K. Micellar structures and their implication in the chemistry and technology of fats and other lipids. Fette Seifen Anstrichm., 88, 79-86 (1986).
- Zhang, F., Köseoğlu, S.S. and Rhee, K.C. Effects of expander process on the phospholipids in soybean oil. J. Am. Oil Chem. Soc., 71, 1145-1148 (1994).
- Cherry, J.P. and Kramer, W.H. Plant sources of lecithin. In: Lecithins – Sources, Manufacture & Uses, pp. 16-31 (B. Szuhaj (ed.), AOCS) (1989).
- Autino, H. Desgomado. In: Temas Selectos en Aceites y Grasas – volumen1 Procesamiento (J.M. Block and D. Barrera-Arellano (eds.), Editorial Blucher, São Paulo, Brasil) (2009).
- Anderson, D. A primer on oils processing technology. In: Bailey’s Industrial Oils & Fats, Sixth Edition, Volume 5, Edible Oil and Fat Products: Processing Technologies, pp. 1-56 (F. Shahidi (ed.), John Wiley & Sons, Hoboken, NJ) (2005).
- Aalrust, E., Beyer, W., Ottofrickenstein, H., Penk, G., Plainer, H. and Reiner, R. (Röhm GmbH and Metallgesellschaft AG), Enzymatic treatment of edible oils, US Patent 5,264,367 (1993).
- Dahlke, K. The enzymatic degumming – EnzyMax. Oléagineux Corps Gras Lipides/OCL, 4,55-57 (1997).
- Dahlke, K. An enzymatic process for the physical refining of seed oils. Chem. Eng. Technol., 21, 278-281 (1998).
- Clausen, I.G., Patkar, S.A., Borch, K., Barfoed, M., Clausen, K., Fuglsang, C.C., Dybdal, L. and Halkier, T. (Novo Nordisk A/S), Method for reducing phosphorus content of edible oils, US Patent 6,103,505 (2000).
- Clausen, I.G., Patkar, S.A., Borch, K., Barfoed, M., Clausen, K., Fuglsang, C.C., Dybdal, L. and Halkier, T. (Novo Nordisk A/S), Method for reducing phosphorus content of edible oils, US Patent 6,143,545 (2000).
- Münch, E.-W. Practical experience of enzymatic degumming. In: Proceedings of the World Conference on Oilseed Processing and Utilization, pp. 17-20 (R.F. Wilson (ed.), AOCS Press, Champaign, IL) (2001).
- Yang, B., Wang, Y.-H. and Yang, J.-G. Optimization of enzymatic degumming process for rapeseed oil. J. Am. Oil Chem. Soc., 83, 653-658 (2006).
- Chakrabarti, P.P., Rao, B.V.S.K., Roy, S.K., Bethala, L.A.P.D., Narayana, P.R.K., Vemulapalli, V., Chelimi, K., Karthika, G., Kale, V. and Prasad, R.B.N. (Council of Scientific and Industrial Research), Process for the pre-treatment of vegetable oils for physical refining, US Patent 7,494,676 (2009).
- Dayton, C.L.G., Staller, K. and Berkshire, T.L. (Bunge Oils, Inc.), Process for improving enzymatic degumming of vegetable oils and reducing fouling of downstream process equipment, US Patent 7,713,727 (2010).
- Gramatikova, S., Hazlewood, G., Lam, D., Barton, N.R., Sturgis, B.G., Robertson, D.E., Li, J., Kreps, J., Fielding, R., Brown, R.C., Vasavada, A., Tan, X., Badillo, A., Van Hoek, W.P., Janssen, G., Isaac, C. and Burk, M.J. (Verenium Corporation), Phospholipases, nucleic acids encoding them and methods for making and using them, US Patent 7,977,080 (2011).
- Dayton, C.L.G. and Galhardo, F. (Bunge Foods Corporation), Enzymatic degumming utilizing a mixture of PLA and PLC phospholipases, US Patent Application Publication 2008/0182322 (2008).
- AB Enzymes Technical Data Sheet. Rohalase® MPL, 2004.
- Maria, L., Vind, J., Oxenbøll, K.M., Svendsen, A. and Patkar, S.A. Phospholipases and their industrial application. Appl. Microbiol. Biotechnol., 74, 290-300 (2007).
- DSM Technical Data Sheet. GumZyme®, 2011.
- Mueller, U., Pascal, G., Olempska-Beer, Z., Leblanc, J.-C. and Meyland, I. Phospholipase-C expressed in Pichia pastoris. In: Safety Evaluation of Certain Food Additives, pp. 107-116. Sixty-ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), Geneva) (2009).
- Galhardo, F. Enzymatic degumming – Recent developments. Paper presented at the AOCS Centenary Meeting & Expo, Orlando, page 120 in the abstracts (2009).
- Galhardo, F. et al. Desgomado Enzimático de Aceites de Vegetales. Grasas Aceites, 79, 204-212 (2010).
Related Resources
Lipid Library
Edible Oil Processing
In the present context, the term edible oil processing covers the range of industrial…
Lipid Library
The Highs and Lows of Cannabis Testing
October 2016 With increasing legalization of both adult recreational and medical cannabis,…
Lipid Library
The secrets of Belgian chocolate
By Laura Cassiday May 2012 Like a bonbon nestled snugly in a…