Separation and Quantification of Oxidized Monomeric, Dimeric and Oligomeric Fatty Acids
The Author: Gloria Márquez-Ruiz, Instituto de Ciencia y Tecnología de Alimentos y Nutrición (ICTAN-CSIC), José Antonio Novais, 10, 28040 Madrid, Spain. DOI: 10.21748/lipidlibrary.39201
1. Introduction
Quantification of the main groups of fatty acids undergoing thermal or oxidative degradation by adsorption and exclusion chromatography following derivatization of oil to fatty acid methyl esters (FAMEs) is the third level for objective and reliable analysis of used frying fats, after evaluation of the total new compounds formed by adsorption chromatography [1] and separation and quantification of the main groups of new compounds, i.e. polymeric triacylglycerols, oxidized monomeric triacylglycerols, diacylglycerols and fatty acids by size exclusion chromatography [2-4].
The analytical procedure was developed in our lab with the aim of acquiring more specific information on frying oil alteration. The first step is derivatization of oil to FAMEs. Although the structure of the triacylglycerol (TG) molecules is lost, the great advantage of transesterification is allowing concentration of exclusively those fatty acyls included in TG molecules bearing oxidized or polymeric functions. The fraction of concentrated polar FAME is separated by adsorption chromatography and further analyzed by high-performance size-exclusion chromatography (HPSEC) for quantification of specific groups of oxidized and polymerized FAMEs [5].
In this article, the basis of the analytical procedure and their main applications in used frying oils and fats will be described.
2. Analytical Procedures
FAMEs are obtained by transesterification of 1 g of oil sample with sodium methoxide and hydrochloric acid-methanol. FAMEs are quantitatively recovered and separated by silica column chromatography in two fractions. A chromatographic glass column of 21 mm internal diameter and 450 mm length is used. Alternatively, smaller quantities of initial oil (200-300 mg) can be separated by adsorption chromatography using silica minicolumns provided that the polar FAME fraction obtained allows gravimetric determination, i.e. when the used frying oil shows relatively high levels of alteration. Thus, a chromatographic glass column of 10 mm internal diameter and 150 mm length can be used. Depending on the objective of the application, two possibilities for fractions separation have been proposed:
- When using light petroleum/diethyl ether 95:5 (150 mL, or 50 mL in minicolumns), the first fraction includes exclusively the most abundant nonoxidized FAMEs. Then, elution with 150 mL diethyl ether yields a minor polar fraction including three groups of FAMEs (oligomers, total dimers and oxidized monomers) (Fig. 1).
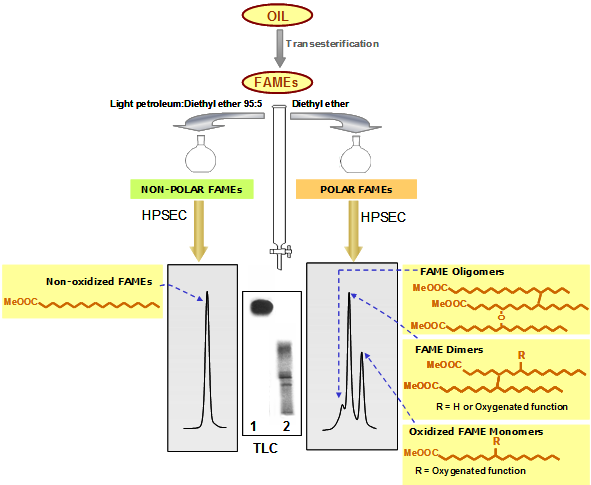
Figure 1. Schematic representation of the methodology based on separation of FAMEs by adsorption chromatography (light petroleum:diethyl ether 95:5 for the first fraction) and HPSEC.
- When using light petroleum/diethyl ether 88:12 (150 mL, or 50 mL in minicolumns) for elution of the first fraction, the combined chromatographic analysis permits the separation of two types of FAME dimers (Fig. 2). Thus, both nonoxidized FAMEs and the nonpolar or nonoxidized FAME dimers, linked by C-C bonds and lacking extra oxygenated functions in their structure, are quantified in the first fraction. Nonpolar dimers are related to thermal degradation and present even in unused frying oils due to refining conditions. FAME oligomers, oxidized FAME dimers and oxidized FAME monomers are determined in turn in the polar fraction. Oxidized FAME dimers are those linked mostly by C-O-C and/or with oxygenated functions in their structure. Therefore, global quantification of the compounds eluted in the second fraction provides a measurement of the total oxidized fatty acyl groups included in TG molecules.
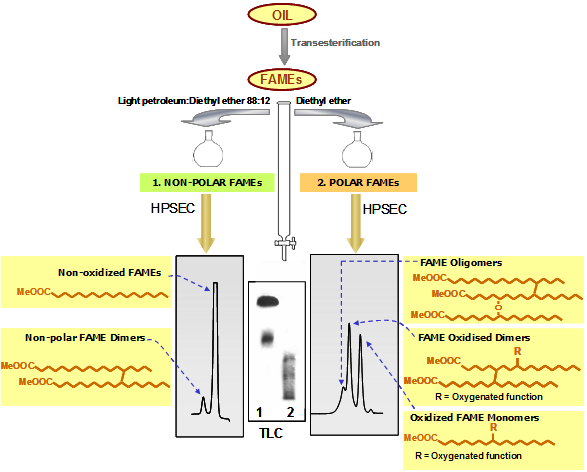
Figure 2. Schematic representation of the methodology based on separation of FAMEs by adsorption chromatography (light petroleum:diethyl ether 88:12 for the first fraction) and HPSEC.
The efficacy of the separation is assessed by thin-layer chromatography (TLC). After gravimetric determination of polar FAMEs, this fraction is further analyzed by HPSEC, using 100 and 500 Å columns with polystyrene divinylbenzene highly cross-linked macroporous packing (particle size: 5 μm) connected in series; mobile phase: tetrahydrofuran (flow rate: 1 mL/min); detector: refractive index. As a result of the combined separation criteria used, firstly based on polarity and secondly on molecular size or weight, nonoxidized, oxidized monomeric, dimeric (nonpolar and oxidized in the case of separation 2) and oligomeric FAMEs are neatly separated and quantified [5].
In Figures 1 and 2, a simplistic structural example is shown in each group of compounds. In reality, each peak includes a high number of different structures. For example, the diversity in fatty acid composition plus the nature, location and number of oxygenated functions formed give rise to a multitude of compounds included in the oxidized monomeric peak. In the case of dimers and oligomers, complexity evidently increases in terms of possible combinations and number of oxygenated functions in addition with the occurrence of C-C or C-O-C linkages in different chain locations.
3. Applications
Analysis of used frying fats and oils
Application of HPSEC to the concentrated fractions of FAMEs has contributed notably to studies of the composition of the polar fraction of used frying oils [5-7].
Table 1 shows data obtained in used frying oils of different alteration levels and three unused oils, selected to illustrate profiles of compounds obtained. Evaluation of samples included both the silica column-HPSEC procedure to determine polar compounds and distribution in oxidized monomeric, dimeric and oligomeric triacylglycerols and the procedure described here (transesterification, separation by adsorption chromatography using separation 2, and analysis by HPSEC) to determine total polar FAMEs and distribution in oxidized monomeric, nonpolar dimeric, oxidized dimeric and oligomeric FAMEs. Differences observed in polar compounds are commented upon in Table 1 of our webpage on Determination of Oxidized Monomeric, Dimeric and Oligomeric Triacylglycerols; Diacylglycerols and Free Fatty Acids.
| Table 1. Polar compounds and polar FAMEs in unused and used frying oils | ||||||||||||||||||
| Sample | Polar compounds (TG) (wt% on oil) |
Polar FAMEs (wt% on FAMEs) |
||||||||||||||||
| Total | Oligomers | Dimers | Oxidized monomers |
Total | Oligomers | Nonpolar dimers |
Oxidized dimers |
Oxidized monomers |
||||||||||
| Unused olive oil | 2.8 | nd | nd | 0.6 | nq | nq | nq | nq | nq | |||||||||
| Unused sunflower oil | 3.4 | nd | 0.9 | 1.6 | 0.8 | nd | 0.3 | nd | 0.5 | |||||||||
| Unused soybean oil | 5.1 | nd | 0.5 | 2.9 | 1.2 | nd | 0.2 | nd | 1.0 | |||||||||
| Used frying oil A | 14.5 | 1.1 | 5.4 | 5.6 | 4.0 | 0.3 | 0.8 | 1.0 | 1.9 | |||||||||
| Used frying oil B | 17.4 | 2.1 | 7.7 | 5.9 | 6.5 | 0.7 | 2.1 | 1.6 | 2.1 | |||||||||
| Used frying oil C | 25.5 | 4.7 | 9.0 | 8.6 | 10.4 | 1.1 | 3.1 | 2.8 | 3.4 | |||||||||
| Used frying oil D | 27.6 | 6.5 | 10.9 | 7.2 | 11.3 | 1.1 | 3.8 | 2.7 | 3.7 | |||||||||
| Used frying oil E | 27.6 | 3.7 | 7.3 | 9.4 | 8.7 | 0.5 | 1.6 | 2.8 | 3.8 | |||||||||
| Used frying oil F | 30.8 | 5.9 | 9.8 | 11.5 | 13.2 | 1.3 | 3.9 | 4.2 | 3.8 | |||||||||
| nd = not detectable; nq = not quantifiable. | ||||||||||||||||||
Unused oils had very low levels of total polar FAMEs, not even quantifiable by gravimetric determination in the case of olive oil and showing poor repeatability in sunflower and soybean oils. Samples of used frying oil (A and B) with polar compoundslevels far below the limit for rejection (25.5-27.6% polar compounds) already contained significant levels of polar FAMEs. Those samples around the limit for rejection gave values of total altered FAME from 8.7 to 11.3% and, among them, substantial amounts of oxidized FAME monomers (34-38 mg/g oil) [6]. In this group, a wide array of oxygenated compounds of unknown nutritional implications is included [8,9].
Samples D (sunflower oil) and E (olive oil), with the same level of polar compounds (27.6%), showed a different pattern of polar compound contribution, with greater polymerization in the case of sunflower oil. This is also and better reflected in the content of total polar FAMEs, specifically in the amounts of oligomeric and nonpolar dimeric FAMEs.
Overall, results revealed some insight into the complexity of the TG oligomers structure, by comparing TG and FAME dimers and oligomers values. In general, the low FAME oligomers-to-TG oligomers ratios in contrast to the FAME dimers-to-TG dimers ratios gave evidence of the considerable contribution of dimeric linkages to the structures of trimeric and higher oligomeric TG [6].
Analysis of oxidized and polymeric fatty acids in digestibility studies
Application of HPSEC to the concentrated fractions of FAMEs has offered an excellent tool to examine digestibility coefficients of five groups of fatty acids originally esterified in TG molecules [10-12] on the basis that the products of lipid digestion ultimately absorbed are largely the fatty acids released by pancreatic lipase. Following analysis of dietary thermoxidized oils and fecal lipids, high digestibility coefficients, averaging 80%, were found for oxidized fatty acid monomers, thus indicating that such oxidized compounds are of the utmost importance from the nutritional standpoint, supported also by their quantitative relevance in the diet. Among polymeric fatty acids, the lowest digestibilities were found for nonpolar dimers, whereas oxidized dimers and oligomers possessed higher apparent absorbability than expected. This could be in part due to depolymerization reactions occurring under the strongly acidic conditions in the stomach.
Results on real used frying samples [6] (examples in Table 1) have shown that fatty acid dimers and oligomers, of low digestibility, were predominantly formed around the limit for fat rejection (25% polar compounds). Concomitantly, substantial amounts of oxidized fatty acid monomers, normally about 30 mg/g oil, were found. Considering that such oxidized monomeric fatty acids globally showed high digestibility coefficients, efforts are being made to identify and quantify specific structures included in this group (see web page - Analysis of Oxidized Fatty Acids).
Interestingly, digestibility of nonoxidized fatty acids was negatively dependent on the global alteration level of the dietary oil. This finding was attributed to impaired hydrolysis of TG oligomers and dimers which include in part nonoxidized fatty acids, due to the difficulties involved in the action of pancreatic lipase on complex glyceridic molecules [13,14]. Also, analyses of fecal lipids confirmed that a considerable fraction of the high MW compounds ingested remained nonhydrolysed [11].
Others
Separation of polar FAMEs has been also used as a preparative technique to concentrate oxidation compounds for analysis of specific structures by GC-MS and GC-FID. Thus, a further step in the evaluation of heated or used frying fats can be achieved, focused on the structures and amounts of oxidation compounds which are easily absorbed and quantitatively relevant. So far, identification and accurate quantification of epoxy, hydroxyl and keto acids, the major fatty acids formed through hydroperoxide decomposition, and short-chain compounds originally linked to the glyceridic backbone, have been approached (see web page - Analysis of Oxidized Fatty Acids).
References
- IUPAC. Standard Method 2.507: Determination of Polar Compounds in Frying Fats. In: Standard Methods for the Analysis of Oils, Fats and Derivatives, 7th ed. (ed. International Union of Pure and Applied Chemistry, Blackwell, Oxford) (1987).
- Dobarganes, M.C., Pérez-Camino, M.C. and Márquez-Ruiz, G. High performance size exclusion chromatography of polar compounds in heated and non-heated fats. Fat Sci. Technol., 20, 308-311 (1988).
- Dobarganes, M.C., Velasco, J. and Dieffenbacher, A. Determination of polar compounds, polymerized and oxidized triacylglycerols and diacylglycerols in oils and fats. Pure Appl. Chem., 72, 1563-1575 (2000).
- Márquez-Ruiz, G., Jorge, N., Martín-Polvillo, M. and Dobarganes, M.C. Rapid, quantitative determination of polar compounds in fats and oils by solid-phase extraction and size-exclusion chromatography using monostearin as internal standard. J. Chromatog. A, 749, 55-60 (1996).
- Márquez-Ruiz, G., Pérez-Camino, M.C. and Dobarganes, M.C. Combination of adsorption and size-exclusion chromatography for the determination of fatty acid monomers, dimers and polymers. J. Chromatogr. A, 514, 37-44 (1990).
- Márquez-Ruiz, G., Tasioula-Margari, M. and Dobarganes, M.C. Quantitation and distribution of altered fatty acids in frying fats. J. Am. Oil Chem. Soc., 72, 1171-1176 (1995).
- Jorge, N., Guaraldo-Goncalves, L.A. and Dobarganes, M.C. Influence of fatty acid composition on the formation of polar glycerides and polar fatty acids in sunflower oils heated at frying temperatures. Grasas Aceites, 48, 17- 24 (1997).
- Márquez-Ruiz, G. and Dobarganes, M.C. Nutritional and physiological effects of used frying oils and fats. In: Deep Frying: Chemistry, Nutrition and Practical Applications – 2nd Edition, pp. 173-204 (ed. M.D. Erickson, AOCS Press, Champaign) (2007).
- Dobarganes M.C. and Márquez-Ruiz, G. Oxidized fats in foods. Curr. Opin. Clin. Nutr. Metabol. Care, 6,157-163 (2003).
- Márquez-Ruiz, G., Pérez-Camino, M.C. and Dobarganes, M.C. Digestibility of fatty acid monomers, dimers and polymers in the rat. J. Am. Oil Chem. Soc., 69, 930-934 (1992).
- Márquez-Ruiz, G., Pérez-Camino, M.C. and Dobarganes, M.C. Evaluation of hydrolysis and absorption of thermally oxidized olive oil in non-absorbed lipids in the rat. Ann. Nutr. Metab., 37,121-128 (1993).
- Márquez-Ruiz, G. and Dobarganes, M.C. Assessments on the digestibility of oxidized compounds from [1-14C]-linoleic acid using a combination of chromatographic techniques. J. Chromatogr. B, 675, 1-8 (1995).
- Márquez-Ruiz, G., Pérez-Camino, M.C. and Dobarganes, M.C. In vitro action of pancreatic lipase on complex glycerides from thermally oxidized oils. Fat Sci. Technol., 94, 307-312 (1992).
- Márquez-Ruiz, G., Guevel, G. and Dobarganes, M.C. Application of chromatographic techniques to evaluate enzymatic hydrolysis of oxidized and polymeric triglycerides by pancreatic lipase in vitro. J. Am. Oil Chem. Soc., 75, 119-126 (1998).
In This Section
- Oil Refining
- Action of Natural Antioxidants During Frying
- Formation of New Compounds During Frying - General Observations
- Formation of cyclic fatty acids during frying
- Formation of Epoxy-, Keto- and Hydroxy-Fatty Acids
- Formation of Volatiles and Short-Chain Bound Compounds
- Formation of Dimers and Oligomers
- Oxysterol Formation Frying Oils
- Structural Analysis of the Cyclic Fatty Acids Formed during Frying
- Cyclic Fatty Acids: Isolation and Quantitative Analysis in Food and Biological Tissues
- Analysis of Used Frying Oils and Fats by High-Performance Size-Exclusion Chromatography
- Analysis of Trans Polyunsaturated Fatty Acids
- Determination of Polar Compounds in Used Frying Oils and Fats by Adsorption Chromatography
- Determination of Oxidized Monomeric, Dimeric and Oligomeric Triacylglycerols; Diacylglycerols and Free Fatty Acids
- Separation and Quantification of Oxidized Monomeric, Dimeric and Oligomeric Fatty Acids
- Analysis of Oxidized Fatty Acids
- Analysis of Oxidized Sterols in Frying Oils
- Metabolism and Physiological Effects of Cyclic Fatty Acids Formed from Linoleic and alpha-Linolenic Acids during Frying
- Metabolism and Physiological Effects of Cyclic Fatty Acids Formed from Linoleic and alpha-Linolenic Acids during Frying
- Metabolism of Trans Polyunsaturated Fatty Acids Formed during Frying
- Biological Effects of Frying Oils Mediated by the Activation of Peroxisome Proliferator-Activated Receptors (PPAR)
