Oxysterol Formation Frying Oils
The Author: Paresh C. Dutta, Food Chemistry, Department of Food Science, SLU, Uppsala, Sweden. DOI: 10.21748/lipidlibrary.39210
1. Introduction
During frying, a number of complex reactions take place in the oil, which depend not only on the biological composition of the raw material, i.e. the content of precursors, but also on the choice of ingredients and processing conditions (frying method, heating time and temperature used during frying, type of fats and oils used, etc.). Oxidation of food lipids is one of the main causes of deterioration of food quality in terms of sensory and nutritional values. There are many different oxidation products generated from the bulk fats and oils, including volatile- and nonvolatile aldehydes, ketones, and hydroxy-, cyclic-, trans- and short-chain fatty acids [1,2].
Fats and oils contain some minor components other than acyl-lipids such as cholesterol (5-cholesten-3β-ol), phytosterols (plant sterols), tocopherols, etc. Cholesterol and phytosterols are structurally similar unsaturated steroid alcohols. Cholesterol is the main animal sterol, while β-sitosterol, campesterol, stigmasterol, brassicasterol, avenasterol, and stigmasterol are major plant sterols present in vegetable oils at much higher levels than cholesterol is in animal fats. These compounds are susceptible to oxidation following autoxidation, photooxidation and enzymatic pathways [3,4]. However, during frying of foods, oxidation of sterols occurs mainly following the autoxidation pathway.
Mixed foods and biological samples can contain a mixture of both cholesterol oxidation products (COPs) and phytosterol oxidation products (POPs), commonly referred to as sterol oxidation products (SOPs = COPs + POPs). The formation, analysis, occurrence, and biological effects of the COPs have been extensively studied. However, research on these aspects with the POPs is still limited [4,5]. COPs have received much attention due to their biological effects such as cytotoxicity, atherogenicity, interference with sterol metabolism, mutagenicity and carcinogenicity. Limited information is available on the biological effects of POPs and their levels in foods and in human plasma [4,5]. The relationship between the long-term consumption of lipid pero-oxidation products and human health is not clear, but it is generally recognized that overused and abused oils undoubtedly contain oxidized material that, if chronically consumed in large amounts, could pose a human health risk [1] .
2. Formation of Sterol Oxidation Products
Autoxidation of cholesterol and phytosterols is facilitated by many factors, for example, temperature, light, oxygen, free radical initiators, metal ions, pro-oxidizing agents, and a shortage of antioxidants. Oxidation mechanisms of phytosterols are believed to follow the same pathways as cholesterol oxidation. Formation of different oxysterols by autoxidation, as documented by experiments, proceeds through a free radical chain reaction. The process is initiated by abstraction of hydrogen from allylic C7 in the ring structure of cholesterol and tertiary carbons at the C20 and C25 positions. The initiation reaction, that is, the formation of free radicals, is not well understood. It is speculated, however, that some probable causative factors are nitrogen oxides, excited oxygen species, or transition metal ions that act as initiation catalysts [4,6]. The radicals thus formed react with oxygen to produce corresponding peroxyl radicals, which in turn are stabilized by yielding different cholesterol hydroperoxides. The thermal decomposition of these hydroperoxides produces 7α-hydroxy-, 7β-hydroxy-, 7-keto-, 20-hydroxy-, and 25-hydroxycholesterol. Epimeric 7-hydroperoxides of cholesterol can also attack the Δ5 double bond of cholesterol, forming secondary oxidation products of cholesterol, such as epimeric epoxycholestanol. Both epoxides, in turn are converted to 5α-cholesta-3β,5,6β-triol, through epoxy ring opening by hydration [6].
Similar oxidation products from phytosterols and their occurrence in foods have been reported in the literature [4]. Concerning oxysterols in fat and oil used for frying and cooking, only six to eight components are generally reported [7,8]. Formation of the main ring structure oxidation products are shown in Figure 1 with the example of sitosterol.
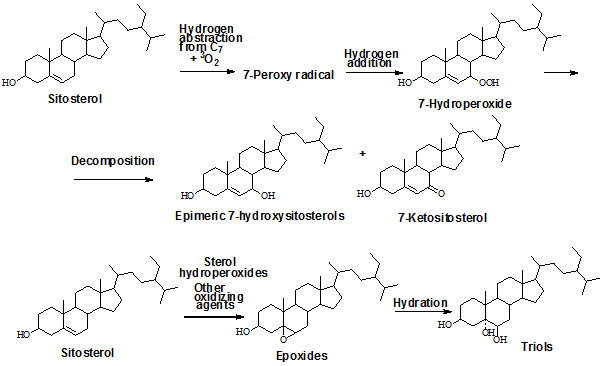
Figure 1. Formation of the main ring structure oxidation products from sitosterol following the autoxidation pathway. Similar oxidation products can be formed from cholesterol and other phytosterols.
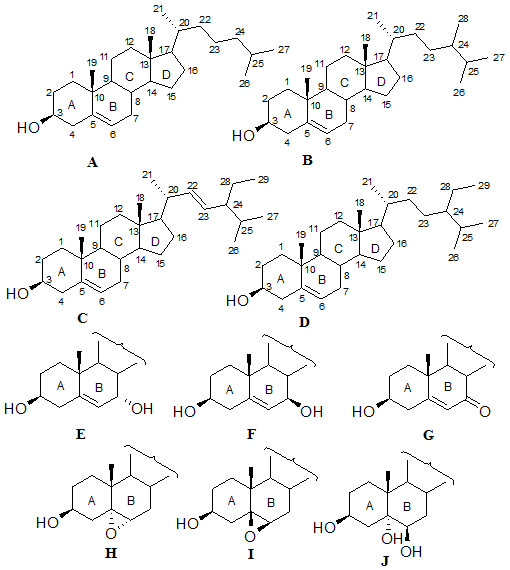
Studies on phytosterol oxidation have focused mainly on sitosterol [9]; however, oxidation of stigmasterol and Δ5-avenasterol has also been reported. Gordon and Magos [10] isolated some oxidation products of Δ5-avenasterol produced by heat treatment at 180°C of pure triacylglycerols containing 0.1% Δ5-avenasterol. After isolation of unsaponifiables and enrichment by preparative TLC, products of oxidized Δ5-avenasterol, such as epimeric 7-hydroxy-, 7-ketoavenasterol, cholesta-3,5-diene-7-one, epimeric 5,6-epoxide, cholest-5-en-3-one, cholest-4-en-3-one, and cholesta-4,6-diene-3-one were confirmed by UV spectroscopy and gas chromatography-mass spectrometry (GC-MS). Some of these compounds are illustrated in Figure 2.
Figure 2. Structures of some common sterols (cholesterol and phytosterols) and their ring structure oxidation products. A, cholesterol; B, brassicasterol; C, stigmasterol; D, sitosterol; E, 7α-hydroxysterol; F, 7β-hydroxysterol; G, 7-ketosterol; H, 5α,6α-epoxysterol; I, 5β,6β-epoxysterol; J, 3β,5α,6β-steroltriol.
Oxidation of stigmasterol by heating purified triacylglycerol containing 5% stigmasterol at 180°C was studied [11]. Using thin-layer chromatography (TLC), and UV and IR spectroscopy, the authors tentatively identified epimeric 7-hydroxystigmasterol, 5,6-epoxide, and stigmast-22-en-3,5,6-triol. Other oxidation products of stigmasterol were characterized by MS, including stigmasta-4,22-diene-3-one, stigmasta-3,5,22-triene-7-one, and stigmasta-3,5,22-triene. A list of the names of some common sterol oxidation products are compiled in Table 1.
| Table 1. Trivial names, abbreviations and systematic names of some common sterols and oxysterols. | ||
| Trivial name | Abbreviation | Systematic name |
| Sitosterol | (24R)-Ethylcholest-5-en-3β-ol | |
| 7α-Hydroxysitosterol | 7α-HSito | (24R)-Ethylcholest-5-en-3β,7α-diol |
| 7β-Hydroxysitosterol | 7β-HSito | (24R)-Ethylcholest-5-en-3β,7β-diol |
| 7-Ketositosterol | 7-KSito | (24R)-Ethylcholest-5-en-3β-ol-7-one |
| Sitosterol-5α,6α-epoxide | α-SitoE | (24R)-5α,6α-Epoxy-24-ethylcholestan-3β-ol |
| Sitosterol-5β,6β-epoxide | β-SitoE | (24R)-5β,6β-Epoxy-24-ethylcholestan-3β-ol |
| Sitostanetriol | SitoT | (24R)-Ethylcholestan-3β,5α,6β-triol |
| 25-Hydroxysitosterol | (24R)-Ethylcholest-5-en-3β,25-diol | |
| Stigmasterol | (24S)-Ethylcholest-5,22-dien-3β-ol | |
| 7α-Hydroxystigmasterol | 7α-HStig | (24S)-Ethylcholest-5,22-dien-3β,7α-diol |
| 7β-Hydroxystigmasterol | 7β-HStig | (24S)-Ethylcholest-5,22-dien-3β,7β-diol |
| 7-Ketostigmasterol | 7-KStig | (24S)-Ethylcholest-5,22-dien-3β-ol-7-one |
| Stigmasterol-5α,6α-epoxide | α-StigE | (24S)-5α,6α-Epoxy-24-ethylcholest-22-en-3β-ol |
| Stigmasterol-5β,6β-epoxide | β-StigE | (24S)-5β,6β-Epoxy-24-ethylcholest-22-en-3β-ol |
| Stigmastentriol | StigT | (24S)-Ethylcholest-22-en-3β,5α,6β-triol |
| 25-Hydroxystigmasterol | (24S)-Ethylcholest-5,22-dien-3β,25-diol | |
| Campesterol | (24R)-Methylcholest-5-en-3β-ol | |
| 7α-Hydroxycampesterol | 7α-HCam | (24R)-Methylcholest-5-en-3β,7a-diol |
| 7β-Hydroxycampesterol | 7β-HCam | (24R)-Methylcholest-5-en-3β,7β-diol |
| 7-Ketocampesterol | 7-KCam | (24R)-Methylcholest-5-en-3β-ol-7-one |
| Campesterol-5α,6α-epoxide | α-CamE | (24R)-5α,6α-Epoxy-24-methylcholestan-3β-ol |
| Campesterol-5β,6β-epoxide | β-CamE | (24R)-5β,6β-Epoxy-24-methylcholestan-3β-ol |
| Campestanetriol | CamT | (24R)-Methylcholestan-3β,5α,6β-triol |
| 25-Hydroxycampesterol | (24R)-Methylcholest-5-en-3β,25-diol | |
| Brassicasterol | (24S)-Methylcholest-5,22-dien-3β-ol | |
| 7α-Hydroxybrassicasterol | 7α-HB | (24S)-Methylcholest-5,22-dien-3β,7α-diol |
| 7β-Hydroxybrassicasterol | 7β-HB | (24S)-Methylcholest-5,22-dien-3β,7β-diol |
| 7-Ketobrassicasterol | 7-KB | (24S)-Methylcholest-5,22-dien-3β-ol-7-one |
| Brassicasterol-5α,6α-epoxide | α-BE | (24S)-5α,6αa-Epoxy-24-methylcholest-22-en-3β-ol |
| Brassicasterol-5β,6β-epoxide | β-BE | (24S)-5β,6β-Epoxy-24-methylcholest-22-en-3β-ol |
| Brassicastanetriol | BT | (24S)-Methylcholest-22-en-3β,5α,6β-triol |
| 25-Hydroxybrassicasterol | (24S)-Methylcholest-5,22-dien-3β,25-diol | |
| Cholesterol | ||
| 7α-Hydroxycholesterol | 7α-HC | Cholest-5-en-3β,7α-diol |
| 7β-Hydroxycholesterol | 7β-HC | Cholest-5-en-3β,7β-diol |
| 7-Ketocholesterol | 7-KC | 3β-Hydroxycholest-5-en-7-one |
| Cholesterol-5α,6α-epoxide | α-CE | 5,6α-Epoxy-5α-cholestan-3β-ol |
| Cholesterol-5β,6β-epoxide | β-CE | 5,6β-Epoxy-5β-cholestan-3β-ol |
| Cholestanetriol | CT | 5α-Cholestan-3β,5α,6β-triol |
| 25-Hydroxycholesterol | 25-HC | Cholest-5-en-3β,25-diol |
To study the formation of side-chain autoxidation products, a mixture of the two phytosterols sitosterol and campesterol was oxidized for 72 h at 120°C in an air-ventilated oven [12]. The following oxidation products were identified and characterized: 24-methylcholest-5-en-3β,24-diol, 24-methylcholest-5-en-3β,25-diol, 24-methylcholest-4-en-6α-ol-3-one, 4-methylcholest-4-en-6β-ol-3-one, 24-ethylcholest-5-en-3β,24-diol, 24-ethylcholest-5-en-3β,25-diol, 24-ethylcholest-4-en-6α-ol-3-one, and 24-ethylcholest-4-en-6β-ol-3-one. The authors further characterized three semipolar side-chain oxidation products of stigmasterol, 24-ethylcholest-5,22-dien-3β,25-diol, 24-ethylcholest-5,22-dien-3β,24-diol, and 24-ethyl-5,22-cholestadien-3β-ol-24-one, in addition to its common ring-structure oxidation compounds, by TLC, GC-MS, and nuclear magnetic resonance spectroscopy [13].
Säynäjoki et al. [14] studied photo-oxidation of stigmasterol in the presence of methylene blue as a sensitizer. The results were similar to those of Bortolomeazzi et al. [15]. As soon as photo-oxidation was started 5α-OOH and 6α - and 6β-OOH were formed, and the amount of 7α-OOH began to increase slightly more slowly than that of 5α-OOH. The authors suggested that might be due to the rearrangement of 5α-OOH to 7α-OOH. The authors did not make any effort to detect 7β-OOH because the analysis of hydroperoxides was made immediately after photo-oxidation allowing no time of rearrangement of 7α-OOH to 7β-OOH.
Adding phytosterols at much higher concentrations than endogenous levels at 2.5% in two types of oils differing in fatty acid composition (soybean oil and high-oleic sunflower oil stripped of tocopherols and phytosterols) demonstrated that soybean oil had lower thermal polymerization during heating at 180°C for 8 h in comparison to high-oleic sunflower oil during heating over 12 h. The losses of phytosterols in soybean oil was lower than in high-oleic sunflower oil, suggesting that the decomposition of phytosterols during heating is affected by the fatty acid composition of the frying oils [16]. Addition of antioxidants to retard lipid oxidation is well documented. It has been shown that addition of 0.2% α-tocopherol in refined oil significantly decreased formation of phytosterol oxidation products during heating at 180°C up to 12 h compared with some other vegetable oils [17]. A recent review has compiled data on the occurrence of phytosterol oxidation products in a wide range of samples [18]. In the past decade, animal fats and hydrogenated fats and oils have gradually been replaced with vegetable oils, amongst which palm oil is the most widely used [19]. During frying of animal food products in vegetable oils, exchange of lipids with the frying oil will occur, so the latter may contain both cholesterol and phytosterol oxidation products.
References
- Dobarganes, C. and Márquez-Ruiz, G. Oxidized fats in foods. Curr. Opin. Clin. Nutr. Metab. Care, 6, 157-163 (2003).
- Saguy, I.S. and Dana, D. Integrated approach to deep fat frying. Engineering, nutrition, health and consumer aspects. J. Food Eng., 56, 143-152 (2003).
- Dutta, P.C. Chemistry, analysis and occurrence of phytosterol oxides in foods. In: Plant Sterols as Functional Food Components and Nutraceuticals, pp. 397-417 (ed. by P.C. Dutta, Marcel Dekker, NY) (2004).
- Dutta, P.C., Przybylski, R., Eskin, N.A. and Appelqvist, L.-Å. Formation and analysis and health effects of oxidized sterols in frying fats. In: Deep Frying:Practices, Chemistry and Nutrition. pp. 125-178 (ed. by M.D. Erickson, AOCS Press, IL) (2007).
- Hovenkamp, E., Demonty, I., Plat, J., Lutjohann, D., Mensink, R.P. and Trautwein, E. Biological effects of oxidized phytosterols: A review of the current knowledge. Prog. Lipid Res., 47, 37-49 (2008).
- Smith, L.L. Cholesterol autoxidation 1981-1986. Chem. Phys. Lipids, 44, 87-125 (1987).
- Bascoul, J., Domergue, N., Olle, M. and Crastes de Paulet, A. Autoxidation of cholesterol in tallows heated under deep frying conditions: Evaluation of oxysterols by GLC and TLC-FID. Lipids, 21,383-387 (1986).
- Nourooz-Zadeh, J. and Appelqvist, L.-Å. Cholesterol oxides in Swedish food and food ingredients: Lard and bacon. J. Am. Oil Chem. Soc., 66, 586-592 (1989).
- Daly, G.G., Finocchiaro, E.T. and Richardson, T. Characterization of some oxidation products of β-sitosterol. J. Agric. Food Chem., 31,46-50 (1983).
- Gordon, M.H. and Magos, P. Products from the autoxidation of Δ5-avenasterol. Food Chem., 14, 295-301 (1984).
- Blekas, G. and Boskou, D. Oxidation of stigmasterol in heated triacylglycerols. Food Chem., 33, 301-310 (1989).
- Johnsson, L. and Dutta, P.C. Characterization of side-chain oxidation products of sitosterol and campesterol by chromatographic and spectroscopic methods. J. Am. Oil Chem. Soc., 80, 767-776 (2003).
- Johnsson, L., Andersson, R.E. and Dutta, P.C. Side-chain autoxidation of stigmasterol and analysis of a mixture of phytosterol oxidation products by chromatographic and spectroscopic methods. J. Am. Oil Chem. Soc., 80, 777-783 (2003).
- Säynäjokii, S., Sundberg, S., Soupas, L., Lampi, A-M. and Piironen, V. Determination of stigmasterol primary oxidation products by high-performance liquid chromatography. Food Chem., 80, 415-421 (2003).
- Bortolomeazzi, R., De-Zan, M., Pizzale, L. and Conte, L.S. Mass spectrometry characterization of the 5α-, 7α-, and 7β-hydroxy derivatives of β-sitosterol, campesterol, stigmasterol, and brassicasterol. J. Agric. Food Chem., 47, 3069-3074 (1999).
- Winkler, J.K. and Warner, K. The effect of phytosterol concentration on oxidative stability and thermal polymerization of heated oils. Eur. J. Lipid Sci., 110, 455-464 (2008).
- Tabee, E., Azadmard-Damirchi, S., Jägerstad, M. and Dutta, P.C. Effects of α-tocopherol on oxidative stability and phytosterol oxidation during heating in some regular and high-oleic vegetable oils. J. Am. Oil Chem. Soc., 85, 857-867 (2008).
- Ryan, E., McCarthy, F.O., Maguire, A.R. and O'Brien, N.M. Phytosterol oxidation products: their formation, occurrence, and biological effects. Food Review Intl., 25, 157-174 (2009).
- Tabee, E., Jägerstad, M. and Dutta, P.C. Lipids and phytosterol oxidation products in commercial potato crisps commonly consumed in Sweden. Eur. Food Res. Technol., 227, 745–755 (2008).
In This Section
- Oil Refining
- Action of Natural Antioxidants During Frying
- Formation of New Compounds During Frying - General Observations
- Formation of cyclic fatty acids during frying
- Formation of Epoxy-, Keto- and Hydroxy-Fatty Acids
- Formation of Volatiles and Short-Chain Bound Compounds
- Formation of Dimers and Oligomers
- Oxysterol Formation Frying Oils
- Structural Analysis of the Cyclic Fatty Acids Formed during Frying
- Cyclic Fatty Acids: Isolation and Quantitative Analysis in Food and Biological Tissues
- Analysis of Used Frying Oils and Fats by High-Performance Size-Exclusion Chromatography
- Analysis of Trans Polyunsaturated Fatty Acids
- Determination of Polar Compounds in Used Frying Oils and Fats by Adsorption Chromatography
- Determination of Oxidized Monomeric, Dimeric and Oligomeric Triacylglycerols; Diacylglycerols and Free Fatty Acids
- Separation and Quantification of Oxidized Monomeric, Dimeric and Oligomeric Fatty Acids
- Analysis of Oxidized Fatty Acids
- Analysis of Oxidized Sterols in Frying Oils
- Metabolism and Physiological Effects of Cyclic Fatty Acids Formed from Linoleic and alpha-Linolenic Acids during Frying
- Metabolism and Physiological Effects of Cyclic Fatty Acids Formed from Linoleic and alpha-Linolenic Acids during Frying
- Metabolism of Trans Polyunsaturated Fatty Acids Formed during Frying
- Biological Effects of Frying Oils Mediated by the Activation of Peroxisome Proliferator-Activated Receptors (PPAR)