The Use of Lithiated Adducts for Structural Analysis of Acylglycerols by Mass Spectrometry with Electrospray Ionization
The Author: Jiann-Tsyh (Ken) Lin, Western Regional Research Center, Agricultural Research Service, United States Department of Agriculture, 800 Buchanan Street, Albany, California 94710 USA
Vibrational Spectroscopy
Mass spectrometry (MS) is a powerful method for the identification of the structures of acylglycerols including the structures of fatty acids attached to the glycerol backbone. As multiple-stage of up to MS4 was necessary for the identification of fatty acid constituents, ion-trap, not quadrupole, MS has been used [1,2]. HPLC (high-performance liquid chromatography) or UPLC (ultra-performance liquid chromatography) is necessary to purify the sample. The direct infusion of the sample including the LC fractions can be used for the identifications, or LC-MS can also be used.
Various adducts of alkali metal and NH4 cations have been used for the MS identification of acylglycerols. The prominent fragment ions (MS2) of the acylglycerol adducts are from the neutral losses of free fatty acids and/or from the neutral losses of fatty acid salts [1,3,5]. The strength of alkali metal and NH4 cations attached to triacylglycerols is in the order of Li+ > Na+ > K+ > NH4+ [4]. The neutral losses of fatty acid sodium salts are more facile than the neutral losses of fatty acid lithium salts [1]. Since MS of lithiated adducts (not other adducts) can be used for the identification of regiospecific isomers of triacylglycerols (by the loss of an α,β-unsaturated fatty acid specifically at the sn-2 position) [5], as well as the identification of the locations of double bonds in the fatty acid constituents [1], lithiated adducts were chosen. The fragment ions (MS2) of lithiated adducts [M + Li]+ from the neutral losses of free fatty acids [M + Li − RCOOH]+ are either exclusive ions or prominent ions with the minor ions from the losses of fatty acid salts [M + Li − RCOOLi]+ (Fig. 1; [1,5]).
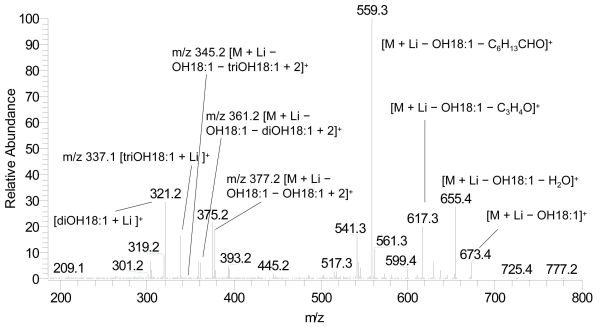
Figure 1. Ion trap mass spectrum of ESI-MS2 of triacylglycerol mixture, diOH18:1-OH18:1-diOH18:1 and triOH18:1-OH18:1-OH18:1, [M + Li]+ at m/z 971.6 in a HPLC fraction of castor oil (collision energy 36%) (Fig. 8 of [7]). Abbreviations: diOH18:1, dihydroxyoleate, triOH18:1, trihydroxyoleate, OH18:1, ricinoleate (hydroxyoleate). C6H13CHO is an aldehyde (for proposed fragmentation pathway see Figure 6A of [6]).
HPLC of Acylglycerols
Reversed-phase (C18) HPLC for the separation of the molecular species of acylglycerols has been reported elsewhere [6,7] and is also reviewed in the Lipid Library. A linear gradient from methanol to isopropanol over 40 min at a flow rate of 1 mL/min has been used in our studies [7]. The eluent used is the least toxic among those used for various HPLC systems of acylglycerols. It is also the least in radioactivity quenching in liquid scintillation counting, so it is ideal for the study of metabolism using radiotracer techniques. A flow scintillation analyzer and UV detector are used simultaneously for the identification of radioactive metabolites. A possible presence of particular molecular species of triacylglycerols in a sample can be elucidated by HPLC using triacylglycerol standards and the HPLC elusion characteristics of the molecular species of triacylglycerols [7].
Preliminary Identification of Acylglycerols
LC-MS will give preliminary identification of the molecular species of acylglycerols in a natural sample. For easier manipulation of the multiple-stage MS for specific molecular species of acylglycerols, we have chosen the direct infusion of the solutions of the HPLC fractions together with lithium acetate into the mass spectrometer and using electrospray ionization (ESI) [2,5]. The solutions of the samples can be in dichloromethane-methanol mixture or in methanol. Lithium acetate can be dissolved in methanol. Triacylglycerols, diacylglycerols and fatty acids will be detected as lithiated adducts in the mass spectra before (MS1) and after fragmentations (MSn, n = 2, 3, 4). MS2 is the fragmentation of a specific mass-to-charge ratio (m/z, usually the charge is one) on a non-fragmented MS1 spectrum. A specific m/z value on an MS1 spectrum may represents more than one lithiated adduct of intact molecular species of acylglycerols in a sample. The MS2 of triacylglycerols (first fragmentation) will give the lithiated positive ions of triacylglycerols after the neutral losses of free fatty acids, [M + Li − RCOOH]+, as shown in Figure 1. The neutral losses of free fatty acids (MS2) can serve for the preliminary identification of the molecular species of acylglycerols in a sample.
Identification of Fatty Acid Constituents in Acylglycerols
The structures of fatty acids attached to the glycerol backbone can be elucidated from the mass spectra of lithiated fatty acids, [RCOOH + Li]+. The ions [RCOOH + Li]+ can be found on the MS3 spectra of triacylglycerols (Fig. 2) originating from the ions of MS2 (Fig. 1), the neutral losses of free fatty acids, [M + Li − RCOOH]+.
Figure 2. Ion trap mass spectrum of ESI-MS3 of [M + Li − OH18:1]+ at m/z 673.4 from Figure 1 (Fig. 9 of [7]). For abbreviations see Figure 1. C3H4O is the glycerol backbone (for proposed fragmentation pathway to form an acid anhydride see Fig. 4B and 4C of [3]). “OH18:1 − 2” is the α,β-unsaturated ricinoleate at the sn-2 position (for proposed fragmentation pathway see Fig. 4A of [3]).
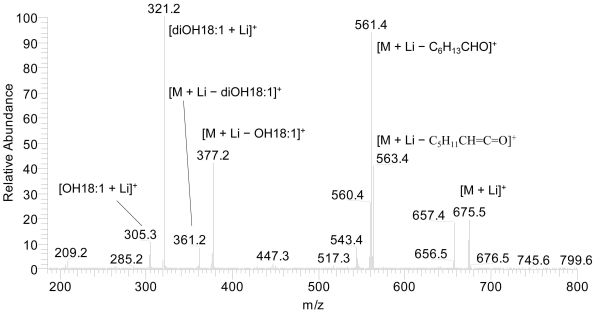
Examples can also be seen in the Figures 1b, 1c, and 1d of [1]. For diacylglycerols, the prominent ions of [RCOOH + Li]+ can be found on the MS2 spectra (Fig. 3). For the identification of fatty acids, the MS spectra of lithiated fatty acids [RCOOH + Li]+ obtained can be compared with those of the standards (commercially available fatty acids) or those that have been published. It might be easier to obtain the mass spectra of fatty acid constituents, [RCOOH + Li]+, from diacylglycerols than from triacylglycerols (Figs. 2, 3).
Figure 3. Ion trap mass spectrum of ESI-MS2 of dihydroxyoleoyl-ricinoleoyl-glycerol [M + Li]+ at m/z 675.5 in a HPLC fraction of castor oil (collision energy 37%) (Fig. 3 of [6]). For abbreviations see Figure 1. C5H11CH=C=O is a ketene (for proposed fragmentation pathway see Fig. 6B of [6]).
Identification and Quantification of Regiospecific Triacylglycerols
Regiospecific triacylglycerols do not differentiate the sn-1 and sn-3 positions, so stereospecific triacylglycerols ABC and CBA are the same regioisomer (A, B and C represent three different fatty acid constituents in triacylglycerols). At this time, MS spectra of stereoisomers of the sn-1 and sn-3 triacylglycerols are identical and cannot be differentiated. The fragment ions from the neutral losses of free fatty acids at the sn-2 position are less abundant than those from the sn-1,3 positions. The linear calibration curves of mixtures of different ratios of regioisomeric standards, AAB and ABA, versus the ratios of ion abundances from the neutral losses of A and B from the mixtures have been used to estimate the ratios of the regiospecific triacylglycerols, AAB and ABA, in natural samples using various MS methods [10,11,12].
Hsu and Turk reported the identification of regiospecific triacylglycerols using the lithiated adducts of triacylglycerols for MS [13]. We have used ESI-MS of lithiated triacylglycerols for the estimation of the ratios of regioisomeric triacylglycerols, AAB and ABA, in castor oil without using regiospecific standards for linear calibration curves [5,14], as regiospecific standards of triacylglycerol containing hydroxy fatty acids were not available. The regiospecific ions used were from the losses of fatty acids as α,β-unsaturated fatty acids specifically at the sn-2 position, [M + Li − R1COOH − R2CH=CHCOOH]+ (MS3) from the MS2, the neutral losses of fatty acid constituents, [M + Li − R1COOH]+. The ratio of the abundances of two regiospecific ions was used to estimate the ratios of AAB and ABA. Figure 2 shows the regiospecific ions from the losses of fatty acids as α,β-unsaturated fatty acids specifically at the sn-2 position. Since Figure 2 was from the mixture of two AAB type triacylglycerols, three regiospecific ions are shown. As the ion m/z 345.2 was not detected, triOH18:1 was not detected at the sn-2 position. For triacylglycerols of ABC type with three different fatty acid constituents using the same method, the ratios of the abundances of the regiospecific ions A:B and B:C at the sn-2 position can be obtained [14]. The ratio of A:B:C at the sn-2 position is about the same as the ratio of the three regioisomeric triacylglycerols.
We have developed a quantification method for the regiospecific isomers of triacylglycerols (AAB and ABA) in a natural sample using one standard AAB without the calibration curve for the mixtures of different ratios of AAB and ABA [15]. Regiospecific AAB includes both stereospecific AAB and BAA. For ABA, the regiospecific ion [ABA + Li − A − B + 2]+ represents the loss of B as α,β-unsaturated fatty acid (B − 2) specifically at the sn-2 position (Fig. 3 of reference [15]). In the same MS spectrum, [ABA + Li − A − A + 2]+ was not detected. This showed that A was not at sn-2 and was at sn-1,3 positions only. For regiospecific AAB, both [AAB + Li − A − A + 2]+ and [AAB + Li − A − B + 2]+ were shown (Fig. 5 of [15]) and the abundance of [AAB + Li − A − A + 2]+ was much more than that of [AAB + Li − A − B + 2]+ (Fig. 5 and Table 1 of [15]). The precursor ion of these two ions was [AAB + Li − A]+ from the neutral loss of A at both the sn-1,3 and sn-2 positions. The ion [AAB + Li − A]+ from the neutral loss of A at the sn-1,3 positions ([ _AB + Li]+) would fragment to [AAB + Li − A − A + 2]+. The ion [AAB + Li − A]+ from the neutral loss of A at the sn-2 position ([A_B + Li]+) would fragment to both [AAB + Li − A − A + 2]+ and [AAB + Li − A − B + 2]+ (see Fig. 4 of [15]).
For the mixture of AAB and ABA as occurring in a natural sample, the abundance of [(AAB + ABA) + Li − A − A + 2]+ represents AAB only and the abundance of [(AAB + ABA) + Li − A − B + 2]+ represents both ABA and small fraction of AAB. This makes the estimation more complicated and need an adjustment to improve the accuracy. The ratio (R, usually 0.2) of the abundances of [AAB + Li − A − B + 2]+ and [AAB + Li − A − A + 2]+ from regiospecific AAB is needed for the adjustment. The total abundance of the ions represents AAB in a sample of the mixture of AAB and ABA is the abundance of [(AAB + ABA) + Li − A − A + 2]+ times (1 + R). The net abundance of the ion represents ABA in a sample of the mixture of AAB and ABA is the abundance of [(AAB + ABA) + Li − A − B + 2]+ minus the fraction (R) of the abundance of [(AAB + ABA) + Li − A − A + 2]+. We assume the ratio of the total abundance of the ions from AAB and the net abundance of the ions from ABA is the same as the ratio of the AAB and ABA contents in a natural sample. We have estimated the ratios of AAB and ABA triacylglycerols in castor oil without this adjustment because of the lack of regiospecific standards, AAB [2,5,14]. This kind of adjustment is not needed for the estimation of the ratio of the three regioisomers of triacylglycerols ABC containing three different fatty acids as shown in reference 14.
References
- Hsu, F.F. and Turk, J. Electrospray ionization multiple-stage linear ion-trap mass spectrometry for structural elucidation of triacylglycerols: Assignment of fatty acyl groups on the glycerol backbone and location of double bond. J. Am. Soc. Mass Spectrom., 21, 657-669 (2010) (DOI: 10.1016/j.jasms.2010.01.007).
- Lin, J.T. and Arcinas, A. Regiospecific identification of 2-(12-ricinoleoylricinoleoyl)-1,3-diricinoleoyl-sn-glycerol in castor (Ricinus communis L.) oil by ESI-MS4. J. Agric. Food Chem., 56, 3616-3622 (2008) (DOI: 10.1021/jf072750z).
- Lin, J.T., Arcinas, A., Harden, L.R. and Fagerquist, C.K. Identification of (12-ricinoleoylricinoleoyl)diricinoleoylglycerol, an acylglycerol containing four acyl chains, in castor oil by LC-ESI-MS. J. Agric. Food Chem., 54, 3498-3504 (2006) (DOI: 10.1021/jf060150e).
- Adams, J. and Gross, M.L. Energy requirements for remote charge site ion decompositions and structural information from collisional activation of alkali metal cationized fatty alcohols. J. Am. Chem. Soc., 108, 6915-6921 (1986) (DOI: 10.1021/ja00282a014).
- Lin, J.T. and Arcinas, A. Regiospecific analysis of diricinoleoylacylglycerols in castor (Ricinus communis L.) oil by electrospray ionization-mass spectrometry. J. Agric. Food Chem., 55, 2209-2216 (2007) (DOI: 10.1021/jf063105f).
- Lin, J.T. and McKeon, T.A. Separation of the molecular species of acylglycerols by HPLC. In: HPLC of Acyl Lipids (Lin, J.T. and McKeon, T.A., eds), H. N. B. Publishing, New York, pp. 199-219 (2005).
- Lin, J.T., Woodruff, C.L. and McKeon, T.A. Non-aqueous reversed-phase high performance liquid chromatography of synthetic triacylglycerols and diacylglycerols. J. Chromatogr. A, 782, 41-48 (1997) (DOI: 10.1016/S0021-9673(97)00482-2).
- Lin, J.T., Arcinas, A. and Harden, L.A. Identification of acylglycerols containing dihydroxy fatty acids in castor oil by mass spectrometry. Lipids, 44, 359-365 (2009) (DOI: 10.1007/s11745-008-3270-6).
- Lin, J.T. and Chen, G.Q. Acylglycerols containing trihydroxy fatty acids in castor oil and the regiospecific quantification of triacylglycerols. J. Am. Oil Chem. Soc., 87, 1371-1379 (2010) (DOI: 10.1007/s11746-010-1617-7).
- Jakab, A., Jablonkai, I. and Forgács, E. Quantification of the ratio of positional isomer dilinoleoyl-oleoyl glycerols in vegetable oils. Rapid Commun. Mass Spectrom., 17, 2295-2302 (2003) (DOI: 10.1002/rcm.1193).
- Byrdwell, W.C. The bottom-up solution to the triacylglycerol lipidome using atmospheric pressure chemical ionization mass spectrometry. Lipids, 40, 383-417 (2005) (DOI: 10.1007/s11745-006-1398-9).
- Leskinen, H., Suomela, J.P. and Kallio, H. Quantification of triacylglycerol regioisomers in oils and fat using different mass spectrometric and liquid chromatographic methods. Rapid Commun. Mass Spectrom., 21, 2361-2373 (2007) (DOI: 10.1002/rcm.3090).
- Hsu, F.-F. and Turk, J. Structural characterization of triacylglycerols as lithiated adducts by electrospray ionization mass spectrometry using low-energy collisionally activated dissociation on a triple stage quadrupole instrument. J. Am. Soc. Mass Spectrom., 10, 587-599 (1999) (DOI: 10.1016/S1044-0305(99)00035-5).
- Lin, J.T. Ratios of regioisomers of triacylglycerols containing dihydroxy fatty acids in castor oil by mass spectrometry. J. Am. Oil Chem. Soc., 86, 1031-1035 (2009) (DOI: 10.1007/s11746-009-1472-6).
- Lin, J.T. and Arcinas, A. Analysis of regiospecific triacylglycerols by ESI-MS3 of lithiated adducts. J. Agric. Food Chem., 56, 4909-4915 (2008) (DOI: 10.1021/jf072837k).
In This Section
- Solid-phase extraction columns in the analysis of lipids
- Preparation of Ester Derivatives of Fatty Acids for Chromatographic Analysis
- Preparation of Lipid Extracts Tissues
- The Chromatographic Resolution of Chiral Lipids
- Detectors for HPLC of Lipids with Special Reference to Evaporative Lght-Scattering Detection
- Why Doesn't Your Method Work When I Try It?
- Laboratory Accreditation in a Lipid Analysis Context
- What Column do I Need for Gas Chromatographic Analysis of Fatty Acids?
- Fatty Acid Analysis by HPLC
- Alternatives to Methyl Esters for GC Analysis of Fatty Acids
- A Practical Guide to the Analysis of Conjugated Linoleic Acid (CLA)
- Application of Infrared Spectroscopy to the Rapid Determination of Total Saturated, trans, Monounsaturated, and Polyunsaturated Fatty Acids
- The Use of Lithiated Adducts for Structural Analysis of Acylglycerols by Mass Spectrometry with Electrospray Ionization
- Identification of FAME Double Bond Location by Covalent Adduct Chemical Ionization (CACI) Tandem Mass Spectrometry
- The Use of Countercurrent Chromatography (CCC) in Lipid Analysis
- Gas Chromatographic Analysis of Plant Sterols
- Analysis of Tocopherols and Tocotrienols by HPLC
- Reversed-Phase HPLC of Triacylglycerols
- Structural Analysis of Triacylglycerols
- Thin-Layer Chromatography of Lipids
- High-temperature Gas Chromatography of Triacylglycerols
- Modification of an AOCS Official Method for Crude Oil Content in Distillers Grains and Other Agricultural Materials
- Lipidomics - A Personal View