Abstract: There has been a tendency to discount thin-layer chromatography (TLC) in recent years because of the availability of high-performance liquid chromatography (HPLC) methods that appeared to offer greater versatility and ease of quantification, amongst other reasons. However, TLC has made a comeback, largely because of the high reproducibility and lower costs of commercial pre-coated plates. It is the quality of the final result that matters and not how it is achieved.
TLC versus HPLC
In recent years, enormous strides have been made in the development of methods for the analysis of lipids using high-performance liquid chromatography (HPLC). It is almost certainly true to say that this technique has replaced preparative gas chromatography entirely. In addition, it could be claimed that there is no type of lipid separation for which thin-layer chromatography (TLC) was once favoured that cannot now be done by HPLC. The latter offers great versatility in that it can be used in the adsorption, reversed-phase, ion exchange and silver ion modes. It operates at room temperature, so is particularly suited to molecules containing thermally labile functional groups. A host of bonded phases, offering varying selectivities in specific analyses, are available commercially and many have yet to be properly explored in lipid applications. Many years ago, I used my TLC spreader for the last time and gave it away to a colleague together with plates, tanks, spray guns and so forth. There must be many other laboratories where this has occurred also. Does this mean that the technique of TLC can now be considered obsolete?
There was a time when I would probably have answered ‘yes’ to this question, but now I must definitely say ‘no’! I doubt whether I will ever go back to making my own TLC plates, in spite of the variety this offers in terms of thickness of layers and the capacity to incorporate complexing agents, such as silver nitrate, into the adsorbent, not to mention costs. Freedom from silica gel dust in the laboratory has turned out to be a considerable boon, and I would be reluctant to return to the times when a grey patina covered all the glassware and furniture in the laboratory. On the other hand, I now use commercial pre-coated plates. HPLC is a superb tool in research and micropreparative applications, but it also has high capital and running costs. Lack of a true and reliable universal HPLC detector rules out the technique for many routine analytical purposes. Higher standards in sample preparation may be necessary than with TLC, for example to eliminate minute particulate contaminants.
At first, commercial pre-coated plates seemed expensive – a rich man’s luxury. Relatively speaking, they do not seem as costly now as they were formerly. We were lured into using them again, because of their convenience, for example in checking purity of a single sample where it was tedious, time-consuming and costly in terms of solvents to do this by HPLC. For this purpose, small pieces of aluminium-backed plates can be used to give an answer in a few minutes. In the analysis of complex lipids, it is a simple matter to use specific spray reagents to detect particular functional groups in lipids separated by TLC, but this is not possible with HPLC.
An unexpected bonus has proved to be that commercial pre-coated plates give much more reproducible results in analysis of complex lipids, especially phospholipids, than was ever possible with laboratory-made plates in the bad old days. With the latter, it was necessary to use silica gel H, i.e. without binder added, which made the layers less stable than was ideal. The brand and even batch of silica gel H seemed to make a great difference to the quality of separation possible; some simply could not be made to work. When a consignment was received that worked well, further material was bought immediately and guarded jealously. Even then, resolution could be very variable and may have been affected by such factors as temperature and humidity, or the presence of differing amounts of ionic species in lipid extracts. It was necessary to be prepared to change mobile phases, apparently arbitrarily, to compensate for these unquantifiable variables. In our experience, this is less of a problem with modern pre-coated plates.
What counts is the quality of the end result and not how it is obtained, and in skilled hands TLC also offers considerable versatility, in that it can be used in adsorption, reversed-phase and complexation modes, and at low cost. In preparative applications, components of interest may be contaminated with silica gel and spray reagents, but there are ways to overcome such problems with a little effort. I do not discuss the IatroscanTM TLC analyser in this brief overview of TLC, as I have no recent experience of the technique; my impression is that it is now little used. Applications of TLC in lipid analysis in general have been reviewed [1-4].
Some Practical Examples
With TLC in the adsorption mode (silica gel), the principal application in lipid analysis is for the separation of different lipid classes from animal and plant tissues. It is a relatively easy matter to resolve each of the main simple lipids from a tissue in one step, i.e. cholesterol esters, triglycerides, free fatty acids, cholesterol and diacylglycerols, using mobile phases consisting of a mixture of hexane and diethyl ether, with a little formic acid to ensure that the free acids migrate successfully. Complex lipids such as phospholipids and glycosphingolipids will remain at the origin, and they can then be quantified as if they were a single lipid class. In the analysis of plasma lipids from clinical experiments, such separations often afford adequate information for diagnostic purposes, and there are many other circumstances where this type of separation is sufficient. It is perhaps one of the disadvantages of HPLC that there is no analogous method for the determination of complex lipids as a single entity.
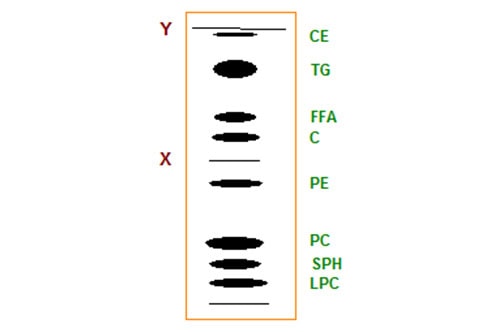
High-performance TLC makes use of silica gel of a very uniform and small particle size, permitting excellent separations with comparatively short elution times. For example, most of the important lipid classes in clinical samples can be achieved by one-dimensional TLC in a single chromatographic run. An example of what can be achieved, taken from the literature, is illustrated in Figure 1 [5].
Figure 1. Schematic separation of simple lipids and phospholipids of plasma by high-performance TLC; two developments as far as “X” with chloroform-methanol-water (60:30:5 by volume), and a third development to “Y” with hexane-diethyl ether-acetic acid (80:20:1.5 by volume). Abbreviations: CE, cholesterol esters; TG, triacylglycerols; FFA, free fatty acids; C, cholesterol; PE, phosphatidylethanolamine; PC, phosphatidylcholine; SPH, sphingomyelin; LPC, lysophosphatidylcholine.
Commercial HP-TLC plates were utilized and they were first given a double development for part of the way in a polar solvent to separate the phospholipids, and then for the full length of the plate with a less polar mobile phase in order to resolve each of the simple lipids. The total elution time was only 30 minutes, in spite of the triple development. In routine analytical work, ten or more samples can be applied to a 20 cm × 20 cm plate and then quantified by charring-densitometry. Although this methodology has been employed mainly in the medical field, I can envisage many similar applications in industrial laboratories.
HP-TLC plates have proved of particular value to research workers who deal with complex glycosphingolipids, such as gangliosides, as the extra resolving power is invaluable. A further advantage in this instance is that analysis times can be reduced from 8-10 to 1-2 hours [6], and that it is possible to use immunological techniques for detection purposes.
Of course, it is possible to isolate the complex lipids from TLC plates and then re-chromatograph them with more polar solvents (and with silica gel without added binder) to isolate each of the individual phospholipid classes, say, with comparable resolution to HPLC.
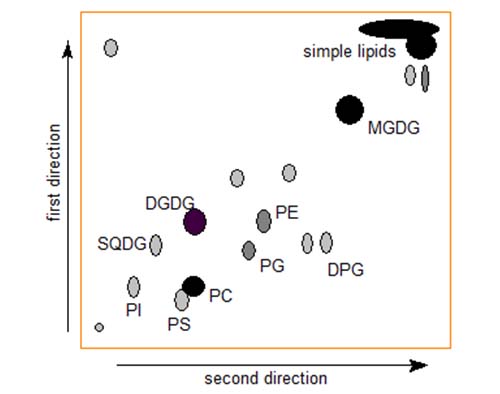
If two-dimensional TLC procedures are used with complex lipids, better resolution may be possible than in a single HPLC run. It should also be noted that we can be sure that we see every lipid in the sample using this technique, but with HPLC it is always possible that some components may be strongly adsorbed and not eluted from the column. Aluminium-backed plates (10 cm × 10 cm) are ideal for analytical purposes with 2-D TLC, but in micropreparative applications (up to 5 mg lipid) glass-backed plates are preferred (20 cm x 20 cm). Figure 2 illustrates the nature of the separation for leaf lipids from Arabidopsis thaliana, a plant species much used in molecular biology. Each of the main individual phospholipids and glycolipids is clearly resolved.
Figure 2. Schematic representation of two-dimensional TLC separation of complex lipids from A. thaliana. Abbreviations, MGDG, monogalactosyldiacylglycerols; DGDG, digalactosyldiacylglycerol; SQDG, sulfoquinovosyldiacylglycerol; DPG, diphosphatidylglycerol; PG, phosphatidylglycerol; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; and PC, phosphatidylcholine.
In this instance, plant phospholipids and glycolipids are separated by first developing the plate in chloroform-methanol-water (75:25:2.5, by volume) in the first direction. After allowing sufficient time for drying, the plate is developed, at right angles to the first development, in chloroform-methanol-acetic acid-water (80:9:12:2, by volume).
For analytical purposes, all components can be detected by spraying the plate with ethanolic phosphomolybdic acid reagent (commercially available as a 20% (w/v) solution), followed by charring in an oven, and they appear as dark spots on a light yellow-green background. We feel that this is less hazardous than the charring reagents containing concentrated sulphuric acid that are often used for universal detection. Alternatively, as an aid to identification, plates can be treated with a variety of specific reagents for different lipid types. Spraying with α-naphthol-sulphuric acid reagent followed by charring is useful for detecting glycolipids, and the phosphate reagent of Dittmer and Lester is specific for the phosphorus moiety in lipids. Ninhydrin reagent shows up lipids containing amino groups, so it is particularly useful for verification of phosphatidylethanolamine and phosphatidylserine. For nondestructive detection of individual polar lipids separated micropreparatively as above, we have found primulin (0.01% (w/v) solution in acetone-water (60:40, v/v) to be preferable to the commonly used dichlorofluorescein or Rhodamine 6G. Lipids are observed under UV light, and give very clear spots even with light spraying with the primulin reagent. The components are extracted from the silica with polar solvent mixtures, prior to analysis by other means.
My colleagues and I have been in the forefront of developing methods for separating complex phospholipids and glycolipids from plant leaf tissue by HPLC methods, and we have used these with thousands of samples for screening purposes. However, when we require a precise detailed analysis of a sample, we go to TLC. This is tedious as it requires that we carry out a separation on the 5 mg scale, locate the spots with the spray reagent, isolate the lipids (with an internal standard added) and then transesterify for gas chromatographic analysis [1]. The important point is that we can get higher accuracy as well as much more information than by HPLC.
I can remember when silver ion TLC was viewed by industrial analysts as a rather esoteric technique, but it has come to be one of the standard methods for the determination of cocoa butter equivalents in confectionery fats, for example. Whether the HPLC or high-temperature gas chromatography methods will supplant it remains to be seen. Silver ion chromatography methods are reviewed in detail elsewhere on this web site.
Reversed-phase TLC has been relatively little used for the analysis of triacylglycerols, mainly because it has been considered a messy technique and because the separated components are not easily visualised. Methods are available which overcome many of these difficulties, and excellent separations of triacylglycerols have been reported [7]. The recommended stationary phase is a silanized kieselguhr, with a mobile phase consisting of acetonitrile-acetone-water mixtures. Charring densitometry is preferred for detection and quantification. However, I have little doubt that in this instance HPLC techniques are much better when the necessary equipment is available.
HPLC linked to electrospray mass spectrometry has made enormous strides forward in the analysis of lipids in recent years (see [1] for a review), but TLC has been used successfully in conjunction with matrix-assisted laser desorption/ionization (MALDI) mass spectrometry [4].
Quantification
The disadvantages of various detectors in HPLC have been debated ad nauseam in recent years. What of quantification in TLC? The generally accepted method for multiple samples involves spraying with a corrosive reagent (usually containing concentrated sulphuric acid), charring at high temperature for a set period, and finally densitometry of the carbon produced. Any experienced analyst coming fresh to lipid methodology would probably look askance at such a procedure. Yet it can be made to work, if great care is taken to standardise the charring conditions and to calibrate for each of the lipid classes with authentic standards, i.e. with a similar degree of unsaturation to the samples. Modern scanning densitometers can give excellent results in skilled hands. However, there is considerable scope for inexperienced analysts to compound the errors. In research applications with relatively few samples, I prefer to transesterify the separated lipids in the presence of an internal standard and use gas chromatography of the methyl ester derivatives of the fatty acids for quantification purposes [1].
Conclusions
What of those of us that have ready access to HPLC? I mentioned earlier that I had resurrected TLC in my own laboratory but with commercial pre-coated plates for the sake of a cleaner environment. TLC has been missed most often when I have had a single sample whose purity or identity needed checking. It is often tedious, time-consuming and costly in terms of solvents to do this by HPLC. In the analysis of complex lipids, it is possible to use specific spray reagents to detect particular functional groups in lipids separated by TLC. I recall listening to a lecture many years ago in which the speaker described a chromatography-tandem mass spectrometry system in which phosphatidylcholine was eventually identified by the presence of a specific ion relating to the choline moiety; the same could have been done with a simple TLC spray. We lack simple colorimetric methods for the identification of components separated by HPLC.
What counts is the quality of the end result and not how it is obtained, and in skilled hands TLC offers considerable versatility and precision in lipid analysis with relatively low capital costs. Of course, there are many parts of the world where HPLC is not available to analysts. If scientists in these countries can obtain equally good results by TLC, they should be encouraged to do so. Suffice it to say that traditional TLC is not dead, but alive and well, and likely to be around for some time to come. A recent review cited earlier makes this point very clearly [4].
References
- Christie, W.W. and Han, X. Lipid Analysis – Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Bridgwater, U.K.) (2010) – www.pjbarnes.co.uk/op/la4.htm.
- Henderson, R.J. and Tocher, D.R. Thin-layer chromatography. In: Lipid Analysis. A Practical Approach (edited by R.J. Hamilton & S. Hamilton, IRL Press, Oxford), pp. 65-111 (1992).
- Touchstone, J.C. Thin-layer chromatographic procedures for lipid separation. J. Chromatogr. B, 671, 169-195 (1995) (DOI: 10.1016/0378-4347(95)00232-8).
- Fuchs, B., Süß, R., Teuber, K., Eibisch, M. and Schiller, J. J. Chromatogr. A, 1218, 2754-2774 (2011) (DOI: 10.1016/j.chroma.2010.11.066).
- Kupke, I.R. and Zeugner, S. Quantitative high-performance thin-layer chromatography of lipids in plasma and liver homogenates after direct application of 0.5-μl samples to the silica-gel layer. J. Chromatogr. B, 146, 261-272 (1978) (DOI: 10.1016/S0378-4347(00)81892-7).
- Yu, R.K. and Ariga, T. Ganglioside analysis by high-performance thin-layer chromatography. Methods Enzymol., 312, 115-134 (2000).
- Amidzhin, B. and Nikolova-Damyanova, B. Densitometric identification of triglycerides separated by reversed-phase thin-layer chromatography. J. Chromatogr. A, 446, 259-266 (1988) (DOI: 10.1016/S0021-9673(00)94441-8).
This article has been updated appreciably from two earlier papers (now amalgamated) by the author that first appeared in Lipid Technology (Christie, W.W. Has thin-layer chromatography had its day? Lipid Technology, 2, 22-23 (1990); Christie, W.W. and Dobson, G. Thin-layer chromatography-revisited. Lipid Technology, 11, 64-66 (1999) (by kind permission of P.J. Barnes & Associates (The Oily Press Ltd.), who retain the copyright to the original articles).
Related Resources
Lipid Library
Edible Oil Processing
In the present context, the term edible oil processing covers the range of industrial…
Lipid Library
The Highs and Lows of Cannabis Testing
October 2016 With increasing legalization of both adult recreational and medical cannabis,…
Lipid Library
The secrets of Belgian chocolate
By Laura Cassiday May 2012 Like a bonbon nestled snugly in a…