Analysis of Tocopherols and Tocotrienols by HPLC
The Author: Anna-Maija Lampi, Department of Food and Environmental Sciences, PO Box 27, Latokartanonkaari 11, FI-00014 University of Helsinki, Finland
1. Introduction
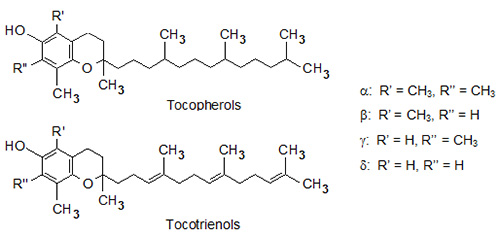
Tocopherols and tocotrienols, collectively known as tocols, are amphipathic and lipid-soluble compounds that are easily oxidized when subjected to heat, light and alkaline conditions [1,2]. They consist of a polar chromanol ring and a hydrophobic 16-carbon side chain attached to the ring via the C-2 atom. Tocopherols have saturated phytyl side chains while tocotrienols have isoprenyl side chains with three double bonds. Both tocopherols and tocotrienols occur as four vitamers (α, β-, γ- and δ-) that differ from each other by the number and position of methyl groups in the chromanol ring (Fig. 1). All natural tocols are 2R-stereoisomers, indicating that the side chain is attached to the chromanol ring with the same stereochemistry [2-4]. Tocopherols contain two additional asymmetric centers in the side chains, namely C-4’ and C-8’, that both are R-stereoisomers in natural vitamers. Double bonds of tocotrienols’ side chains at C-3’ and C-7’ have trans-configuration.
Figure 1. Chemical structures of tocopherols and tocotrienols.
Animals must obtain tocols from their diet, because they are synthesized only in photosynthetic organisms: mainly in chloroplast membranes, chromoplasts and the oil bodies of seeds [5,6]. Tocopherols are widely distributed in higher plants, whereas tocotrienols occur only in some nonphotosynthetic tissues, such as in certain monocot seeds and latex. Therefore, tocopherols are present in most plant foods, and, for example, vegetable oils and nuts are rich in them. Most tocotrienols of the Western diet originate from palm oil and some cereal grains, such as wheat, barley and oats [4].
All tocols were considered to possess the same biological activity of α-tocopherol, i.e. vitamin E activity. The natural α-tocopherol showed the highest activity, while those of β-, γ- and δ-tocopherols and α- and β-tocotrienols were 50%, 10%, 3%, 30% and 5% of that of α-tocopherol, respectively [2,4]. These relative values reflected the greater retention and distribution of α-tocopherol in animals. However, in the year 2000, Vitamin E activity was limited to α-tocopherol with 2R-configuration, because other forms do not convert to α-tocopherol and are poorly recognized by the transfer protein [7]. The main physiological task of 2R-α-tocopherol is to avoid vitamin E deficiency symptoms, i.e. neurological abnormalities. Being antioxidants, all tocols reduce oxidative stress and delay the progress of a variety of degenerative diseases such as cardiovascular diseases and cancer. In addition, they have been shown to regulate cellular signaling, cell proliferation and gene expression [8,9]. Tocotrienols are considered to possess additional positive health effects beyond those of tocopherols including, for example, induction of immune responses and lowering of serum cholesterol levels [8,10].
The most important chemical property of tocols is their antioxidant activity, and all of their biological activities are considered to derive from their ability to protect polyunsaturated lipids from oxidation [9]. Tocols are primary antioxidants that scavenge lipid peroxyl radicals by donating hydrogen atoms, and react with reactive oxygen and nitrogen species [1,11]. Although tocols protect lipids from oxidation by similar reactions in vivo and in vitro, antioxidant activities may differ greatly. In vivo, α-tocopherol appears to be the major and most efficient lipid-soluble chain-breaking antioxidant, mainly because it works as part of an antioxidant network, which includes ascorbic acid, glutathione and ubiquinol, for example. Tocopheroxyl radicals may be regenerated to tocopherols in vivo, while in vitro recycling does not occur. For understanding the antioxidant functions of tocols in food systems, see the recent review by Seppanen et al. [12]
Since the biological activities and chemical properties of tocols differ from each other, it is important to be able to determine each vitamer separately.
2. Sample Preparation
Sample preparation is the most time-consuming step in analysis, and also the main source of error. To reduce the efforts needed and avoid tocol losses, sample workup should be as simple as possible. Since tocols are lipid-soluble compounds they are readily soluble in organic solvents, and solvent extraction has been the most commonly used method to extract tocols from, e.g. oil seeds, biological fluids and animal tissues [3,4,13]. The solvent should be efficient enough to penetrate the water phase to reach tocols, and to liberate them from lipophilic membranes. In cereal grains and several processed foods, tocols are associated with other compounds, such as carbohydrates and proteins, and these interactions need to be broken down prior to extraction. Often this is done by alkaline hydrolysis, i.e. saponification [14-16]. Additionally, the HPLC system may also set special needs for the sample preparation method.
To improve extraction yields from dry materials, samples should be finely ground. For example, tocopherol yields of almond seeds by supercritical carbon dioxide extraction were more than 30-fold greater after grinding the seeds to 0.5-3 mm particle size, and even more than 100-fold greater after being powdered [17]. Since tocols are easily oxidized, it is important to avoid conditions that would promote oxidation during sample preparation or storage. It is recommended to analyze samples freshly after grinding and homogenization or to store them at low temperatures in the dark, and to work under subdued light, and to use inert atmosphere and antioxidants when possible.
2.1 Solvent extraction
Solvent extraction is a simple procedure to extract tocopherols and tocotrienols from tissue and oilseed samples [3,4,13]. The extract may be used directly after being dissolved in the mobile phase and filtered, or after additional purification steps. There are no universal extraction solvents that would yield optimal results in all materials. Instead, extraction parameters should be optimized for each purpose. For example, hexane and a mixture of hexane and ethanol were used to extract tocols from meal and food samples [18,19], a mixture of chloroform and methanol from pumpkin seeds [20], and methanol from cereal grains [21]. Ethanol treatment prior to solvent extraction has also been used to release tocols from proteins in wet tissues [22,23]. Extraction may also be improved for example by vortexing, sonication or using ultrasound [18,23-25], or by using hot circulating solvents such as in Soxhlet extraction [14]. Although solvent extractions are carried out under relatively mild conditions, antioxidants have been added to solvents to protect tocols from degradation during extraction and storage [19,22].
2.2 Alkaline hydrolysis-assisted extraction
Alkaline hydrolysis improves extractability of tocols from hard tissues and complex foods by weakening the interactions between tocols and the matrix, and by softening the matrix [14-16]. For example, alkaline hydrolysis was needed to quantitatively release tocols from margarines and rolling fats [26]. Natural tocols would not need to be hydrolyzed, because they occur mainly as non-conjugated free compounds, but fortified foods and feeds should be saponified, because tocols are generally added as esters. When analyzing tocols by normal-phase HPLC, co-extracting neutral lipids do not interfere the separation [18], but when analyzing tocols by reversed-phase HPLC, alkaline hydrolysis is recommended to remove saponifiable lipids [27,28].
Since tocols are prone to decomposition under alkaline conditions, significant losses may occur unless carefully protected from oxidation. In earlier studies, alkaline hydrolysis was performed at room temperature [18], but more recently faster, efficient and nondestructive methods including hot alkaline hydrolysis have been developed [14,16,29]. To avoid oxidation, antioxidants such as ascorbic acid [18,26] or pyrogallol ]14,15,21,30] are commonly added to the saponification mixture, and oxygen is removed from the vessel by purging the air space with nitrogen. After saponification, tocols are commonly extracted with solvent mixtures such as hexane and ethyl acetate [15,21], heptane and ethyl acetate [16] or hexane and ethanol [29]. The nonsaponifiable lipid extracts are purified from water-soluble impurities, and finally concentrated and filtered prior to HPLC analysis.
Despite the possible risks associated with alkaline hydrolysis in tocol analysis, two studies on extracting tocols from cereal samples clearly showed that it is an efficient and safe means to improve tocol release from complex matrices. Tocol yields from barley samples after hot alkaline hydrolysis were 0-32% and 7-33% greater than by using direct solvent extractions with n-hexane/ethyl acetate and methanol, respectively [15], and from oats approximately 25% greater than by using methanol extraction alone [21].
2.3 Other techniques to extract tocols
As an alternative to traditional solvent extraction methods tocols may be extracted by supercritical fluid (SCF) or pressurized liquid extraction (PLE) techniques. They enable shorter extraction times, decrease solvent volumes and facilitate automation, and are thus suitable to routine work with lots of samples [14,31,32].
SCF is especially environmentally friendly, because little or no organic solvents are used. Extraction parameters, e.g. temperature and fluid density, are easily optimized and managed. Carbon dioxide alone or with methanol as modified were used to extract tocols from low-moisture plant tissues [14,28] and dairy and meat products [33] with only somewhat smaller recoveries than by solvent extraction. Application of SCF to extract tocols from more complex and moist samples such as cheese and salami showed, however, poor extraction efficiency and repeatability [34].
The main advantage of PLE is the possibility to use elevated temperatures to enhance extraction. The main parameters to be optimized include choice of solvent, extraction time and temperature, and amount of sample [32,35]. Hexane, dichloromethane, isopropanol and ethanol gave comparable yields of γ-tocopherol from corn and oats [35]. Methanol was successfully used to extract tocols from cereals and infant formulas at 50°C and a pressure of 110 bar [31,36]. Later, different solvent mixtures were compared to extract tocols from various cereal samples [32]. Acetonitrile was found to be optimal for oats, a mixture of methanol and propanol for palm oil, and methanol for wheat, rye, barley and corn. In general, recoveries of tocols and precisions of extractions are good in PLE, and thus new applications are constantly being developed.
Solid-phase extraction (SPE) has been applied to effectively absorb and concentrate tocols from various matrices. For example, silica cartridges were used to concentrate tocols from extracts [37], and on-line coupling of SPE with HPLC enabled reliable and fast analysis of α-tocopherol and δ-tocopherol together with several other fat-soluble vitamins in serum [38]. More recently tocol-specific polymeric materials have been developed to selectively and accurately bind tocols from leaf and plasma samples [39,40], for example.
By using these alternative extraction techniques it has been possible to work up more samples with good repeatability than by manual extraction, which is vital when the number of samples is large and the analyses are performed regularly, e.g. in screening and follow-up studies. These and also traditional sample preparation methods should be carefully optimized with careful consideration of the chemical and physical properties of the sample matrix and tocols, and validated before they can be used.
3. HPLC Separation of Tocopherols and Tocotrienols
HPLC is the most widely used technique to analyze tocols, and both normal-phase (NP) and reversed-phase (RP) chromatography are applied [3,4,13,41]. Tocols are stable under HPLC conditions, easy to dissolve in appropriate solvents, and there are several detectors that can be combined with HPLC to detect tocols. Fluorescence detection (FLD) and ultraviolet detection (UV) are the most commonly used in food and feed analyses. Gas chromatography (GC) could also be used to analyze tocols, but that would need derivatization of the analytes and might include a risk for decomposition due to high temperatures. Yet GC is still sometimes being used for tocol analysis [2]. When all eight tocols need to be separated, NP-HPLC is commonly applied, but when only some vitamers are of interest, RP-HPLC might also be utilized. RP-HPLC is also used when mixtures of fat-soluble vitamins and free and esterified tocols are to be separated.
In NP-HPLC, the vitamers are dissolved in relatively nonpolar organic solvents and separated by adsorption, which is considered to be the most effective for separating vitamers (e.g. [3,4]). The polarity of tocols is mainly influenced by the number of methyl groups in the chromanol ring, and to a lesser extent by steric effects of the methyl groups and slightly increased polarity of the unsaturated side chains of tocotrienols compared to those of tocopherols [41]. The most difficult compounds to be separated are the β- and γ-tocols, because they have three methyl groups in their ring structure (e.g. [3,13]).
All eight tocols have been base-line separated by NP-HPLC using silica columns (Table 1). Better selectivity was achieved using hexane with a relatively strong polar modifier 1,4-dioxane in the mobile phase than with a weak modifier such as tert-butyl methyl ether [41]. Using 4–5% (v/v) of 1,4-dioxane in hexane as the mobile phase with several silica columns, tocols were always eluted in the following order: α-tocopherol < α-tocotrienol < β-tocopherol < γ-tocopherol < β-tocotrienol < γ-tocotrienol < δ-tocopherol < δ-tocotrienol, and a good separation was achieved.
| Table 1. Examples of HPLC systems to separate and quantify α-, β-, γ-, and δ-tocopherols and α-, β-, γ-, and δ-tocotrienols | |||
| Stationary phase | Mobile phase | Detection | Reference |
|---|---|---|---|
| Silica columns: | |||
| Alltima SI 5U, 5 μm, 250 mm *4.6 mm; | 4-5% 1,4-dioxane in n-hexane, 1.5-2 mL/min | FLD: λEx = 294 nm; λEm = 326 nm | 41 |
| Inertsil silica, 5 μm, 250 mm *4.6 mm; | |||
| Genesis silica, 4 μm, 250 mm *4.6 mm | |||
| Kromasil Phenomenex Si column, 5 μm, 250 mm *4.6 mm | 1.8% ethyl acetate and 0.9% acetic acid in n-hexane, 1.6 mL/min | FLD: λEx = 280 nm; λEm = 325 nm | 14 |
| Kromasil Phenomenex Si, 5 μm, 250 mm *4.6 mm | 1.8% ethyl acetate and 0.9% acetic acid in n-hexane, 1.6 mL/min | FLD: λEx = 290 nm; λEm = 330 nm | 15 |
| Inertsil 5 silica, 5 μm, 250 mm *4.6 mm, 30°C | 3% 1,4-dioxane in n-hexane, 2 mL/min | FLD: λEx = 292 nm; λEm=325 nm | 16 |
| Partisil Pac, 5 μm, 250 mm *4.6 mm, 40°C | 6-12% tetrahydrofuran in hexane, 1 mL/min | FLD: λEx = 295 nm; λEm = 325 nm | 5 |
| Inertsil 5 silica, 5 μm, 250 mm *4.6 mm, 30°C | 3% 1,4-dioxane in n-heptane, 2 mL/min | FLD: λEx = 290 nm; λEm = 325 nm | 26 |
| LiChrosorb Si-60, 5 μm, 125 mm *4 mm, 25°C | 3.6% ethyl acetate and 1.8% acetic acid in hexane, 1.0 mL/min | FLD: λEx = 290 nm; λEm = 330 nm | 25 |
| Supelcosil LC-SI, 3 μm, 75 mm *3 mm, 21°C | 2% 1,4-dioxane in n-hexane, 0.7 mL/min | FLD: λEx = 290nm; λEm = 330 nm | 54 |
| Phenomenex silica, 5 μm, 250 mm *4.6 mm | 4% tetrahydrofuran in hexane, 1 mL/min | FLD: λEx = 290 nm; λEm = 330 nm | 43 |
| Other columns: | |||
| Supelcosil LC-Diol, 5 μm, 250 mm *4.6 mm | 1% 2-propanol in hexane | FLD: λEx = 296 nm; λEm = 330 nm | 18 |
| LiChrosorb Diol Hibar, 5 μm, 250 mm *4mm | 4% tert-butyl methyl ether in n-hexane, 2 mL/min | FLD: λEx = 294 nm; λEm = 326 nm | 41 |
| Hypersil APS-2, 5 μm, 250 mm *4.6 mm (aminopropyl silica) | 5% 1,4-dioxane in n-hexane, 2.5 mL/min | FLD: λEx = 294 nm; λEm = 326 nm | 41 |
| LiChrosorb NH2, 5 μm, 250 mm *4.6 mm | 20% tert-butyl methyl ether, 1% tetrahydrofuran and 0.1% methanol in hexane, 1 mL/min |
FLD: λEx = 294 nm; λEm = 326 nm | 41 |
| Taxil PFD, 3 μm, 250 mm *4.6 mm (pentafluorophenylsilica) | 90% methanol in water, 0.5 mL/min | FLD: λEx = 298 nm; λEm = 345 nm | 30 |
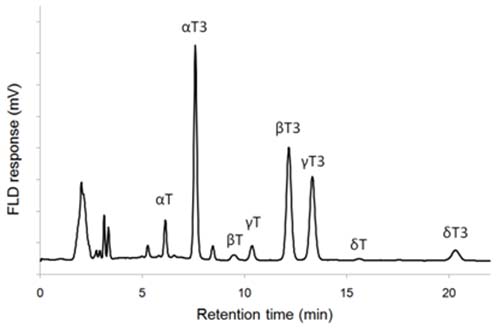
As an example, eight tocols of barley extract were fully separated by using 3% (v/v) of 1,4-dioxane in heptane (Fig. 2). Baseline separation of γ-tocopherol and β-tocotrienol could not be obtained using tert-butyl methyl ether, 2-propanol or diisopropyl ether as the modifier [41]. However, by using eluent mixtures containing 0.9−1.8 % (v/v) of acetic acid in n-hexane and ethyl acetate a nice separation with silica columns was obtained [14,25]. Adequate separation of all tocopherols and most tocotrienols has also been obtained by using 0.5−1 % (v/v) of 2-propanol in hexane or heptane with a silica column [13,44,45]. In addition, diol and amino propyl bonded silica columns have been used to separate tocols [18,41].
Figure 2. NP-HPLC-FLD chromatogram of barley extract. T: tocopherol, T3: tocotrienol (a silica column and 3% (v/v) of 1,4-dioxane in heptane as eluent; for details see ref. 16).
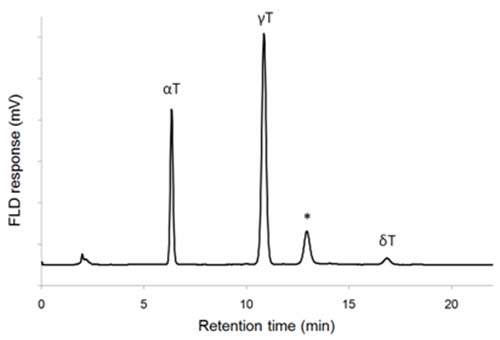
NP-HPLC-FLD chromatograms may have some additional peaks that represent other compounds than the common tocols. One such compound is plastochromanol-8, a homolog of γ-tocotrienol that has eight unsaturated isoprene units in the side chain and a molecular mass of 750. It is found in many vegetable oils [26], and may be incorrectly identified as β-tocopherol or γ-tocotrienol (Fig. 3).
Figure 3. NP-HPLC-FLD chromatogram of rapeseed oil showing elution of plastochromanol-8 (*). T: tocopherol, T3: tocotrienol (a silica column and 3% (v/v) of 1,4-dioxane in heptane as eluent; for details see ref. 16).
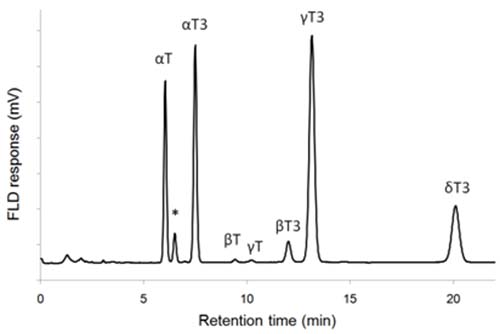
An additional tocol, α-tocomonoenol, has been found in palm oil and pumpkin seeds, and identified by MS and NMR [42,43]. Based on its retention in RP- and NP-HPLC, and molecular mass of 428, it was localized as the peak eluting between α-tocopherol and α-tocotrienol in NP-HPLC (Fig. 4). Another monoenol, γ-tocomonoenol, was also characterized in pumpkin seed extracts, and was eluted between β- and γ-tocotrienols [43].
Figure 4. NP-HPLC-FLD chromatogram of palm oil extract (Tocomin 50, Carotech Inc., Edison NJ) showing elution of α-tocomonoenol (*). T: tocopherol, T3: tocotrienol (a silica column and 3% (v/v) of 1,4-dioxane in heptane as eluent; for details see ref. 16).
RP-HPLC has also been used to analyze tocols. It has the advantage over using NP-HPLC that less hazardous mobile phases, e.g. methanol or ethanol, may be used, but sample preparation needs to include removal of acyl lipids, i.e. saponification, to avoid contamination of the RP column. Complete resolution of the eight tocols has not been obtained with C18-bonded silica columns, because they were not able to separate β- and γ-vitamers from each other [31,46]. In many applications, such a separation is not needed, and thus C18-bonded columns are being used. By using a C30-bonded silica column and methanol as the mobile phase, it was possible to separate α-tocopherol and all tocotrienols in crude palm oil [42]. Another type of RP column, pentafluorophenylsilica (PFPS) column, has also been used to separate tocols from plant seed oils [30] and tocopherols from sunflower oil [27]. The PFPS column with a mobile phase of methanol and water could separate all eight tocols [30]. Recently it has been possible to use Ultra-High-Pressure LC (UPLC) to separate and quantify tocopherols efficiently and fast by RP technique [47,48]. Complete analysis of α-, (β+γ)- and δ-tocopherols by RP-HPLC was obtained in 2-3 min.
Because RP-HPLC is able to separate analytes with greater differences in chemical properties, it has been applied to analyze simultaneously tocols and other fat-soluble compounds. A C18-bonded column with an eluent containing 2.5 mM sodium acetate buffer in methanol-water (94:6, v/v) mixture was used to separate α-, (β+γ)- and δ-tocopherols and retinol and retinyl acetate in infant formulas [36], and another C18-bonded column with an eluent of methanol-water (99:1, v/v) separated α-tocopherol, retinol and retinyl acetate [49]. It was possible to analyze α-, (β+γ)- and δ-tocopherols in a mixture containing also retinol and retinyl and α-tocopheryl esters by a rapid method based on a C18-bonded column and an eluent containing a cationic surfactant [50]. A C30-bonded RP column was used to separate α-, (β+γ)- and δ-tocopherols and other fat-soluble vitamins and carotenoids with gradient elution containing methanol and isopropanol-hexane (50:50, v/v) [51].
4. Detection of Tocopherols and Tocotrienols
Tocols absorb UV light at λ = 290-300 nm, but the maximal absorbances are so small that UV absorption can only be used to detect and quantify tocols in tocol-rich samples such as vegetable oils [44,45]. Better sensitivity and higher selectivity of HPLC analysis is obtained by using FLD and this is the technique of choice for most biological samples. An excitation wavelength of 290-296 nm and an emission wavelength of 325-330 nm are commonly used [18,21,26]. The linearity of calibration curves of tocols using FLD should be evaluated for each analytical method and instrument, because there are significant differences in the linearity ranges; linear ranges from 0.1 to 5 µg/mL (r2 > 0.992) [19] and from 0.01 to 50 µg/g (r2 > 0.995) [52], or from 10 to 100 ng (r2 > 0.99) and from 3-4 ng to 2000 ng (r2 > 0.99) on column [18,26] have been reported. Mass spectrometry (MS) has also been used to detect tocols. Negative ion APCI (atmospheric pressure chemical ionization) was found the best choice among negative and positive ion electrospray and APCI ionization modes to detect and quantify tocopherols [27].
5. Identification and Quantification of Tocopherols and Tocotrienols
Authentic tocopherol standards are commercially available, and thus it is relatively easy to identify them from chromatograms obtained by FLD or UV responses. Elution orders of different tocopherols can also easily be found in literature. Tocotrienol standards, however, are not available as pure compounds, and thus their identification is usually done by using palm oil or cereal grain extracts that are known to contain tocotrienols. LC-MS may also be used to confirm identities of tocols. For example, by using positive APCI, mass to charge ratios (m/z) of [M+H]+ ions were 431, 417, 417, and 403 for α-, β-, δ-, and δ-tocopherols, and 425, 411, 411, and 397 for tocotrienols, respectively. Characteristic fragment ions were m/z 205 and 165 for α-tocols, m/z 191 and 151 for β-and γ-tocols, and m/z 177 and 137 for δ-tocols [53].
Calibration curves of tocopherols should be measured on regular basis. Since pure tocotrienols are not commercially available, their quantification is commonly done by using tocopherols. Fortunately tocotrienols are known to exhibit similar fluorescent responses to their respective tocopherols, and thus tocotrienols may be quantified using tocopherol standards [41,44]. In some studies tocotrienols have been purified from barley or other cereal grains by solvent extraction and preparative HPLC, and used as standards [14,15]. UV absorptivities of pure tocopherols are useful in checking the concentrations of tocopherols in standard stock solutions (Table 2). Tocols are usually quantified using an external standard method, because it is difficult to find a compound that would not interfere with tocol analysis. Examples of possible internal standards include 5,7-dimethyltocol that could be used when the sample did not contain α-tocotrienol [18], and 2-methyl-2-(4’,8’,12’)-trimethyltridecyl)chroman-6-ol that was baseline separated from other tocols [54]. Alternatively, those tocopherols that are not present in the samples may also be used as internal standards.
| Table 2. UV absorptivities of tocols. T: tocopherol, T3: tocotrienol | |||||||||
| α-T | β-T | γ-T | δ-T | α-T3 | β-T3 | γ-T3 | δ-T3 | Reference | |
| λmax, nm | 292.5 | 296 | 298 | 298 | 292.5 | 294 | 296 | 297 | 19, 46 |
| E1% in ethanol |
75.8 | 89.4 | 91.4 | 87.3 | 91 | 87.3 | 90.5 | 88.1 | |
6. Quality Aspects
Validation of a method to analyze tocols in biological samples should be done before each new method application and type of sample is introduced. The performance of the chromatographic system to separate and detect tocols should be evaluated and presented [41,44]. It is more difficult to validate sample preparation, because there are no certified reference materials (CRM) containing a set of natural tocols. Only a few CRMs of materials fortified with α-tocopherol or its esters are available, but they are of minor value when heterogeneous and complex samples are studied. Thus, validation of sample preparation should be done indirectly. Usually this is done by studying recoveries and precisions of spiked tocols, which describe how the added analytes are retained during the whole sample workup and HPLC analysis. This does not, however, give information on how efficiently tocols are released from the sample matrix, which is a major concern. When possible, the performance of a new method should always be compared to a standard method, e.g. EN 12822 [45], or at least to a method that has been commonly used and properly validated. In-house reference samples are valuable in comparing different methods and controlling analytical levels from day to day.
Finally, when choosing and validating a method to analyze tocols, it is good to remember that the method should be fit for purpose, not more – not less.
Acknowledgements: The author would like to thank Ms. Mari Lehtonen for the processing of chromatographic data into figures.
References
- Kamal-Eldin, A. and Appelqvist, L. The chemistry and antioxidant properties of tocopherols and tocotrienols.Lipids, 31, 671-701 (1996).
- Eitenmiller, R.R. and Lee, J. Vitamin E, Food Chemistry, Composition, and Analysis. 540 pp., Marcel Dekker Inc., New York, NY (2004).
- Abidi, S.L. Chromatographic analysis of tocol-derived lipid antioxidants. J. Chromatogr. A, 881, 197-216 (2000).
- Bramley, P.M., Elmadfa, I., Kafatos, A., Kelly, F.J., Manios, Y., Roxborough, H.E., Schuch, W., Sheehy, P.J.A. and Wagner, K.H. Review: Vitamin E. J. Sci. Food Agric., 80, 913-938 (2000).
- Horvath, G., Wessjohann, L., Bigirimana, J., Jansen, M., Guisez, Y., Caubergs, R. and Horemans, N. Differential distribution of tocopherols and tocotrienols in photosynthetic and non-photosynthetic tissues. Phytochemistry, 67, 1185-1195 (2006).
- Hunter, S.C. and Cahoon, E.B. Enhancing vitamin E in oilseeds, Unraveling tocopherol and tocotrienol biosynthesis. Lipids, 42, 97-108 (2007).
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoid, 506 pp., National Academy Press, Washington, DC (2000).
- Sen, C.K., Khanna, S. and Roy, S. Tocotrienols in health and disease, The other half of the natural vitamin E family. Mol. Aspects Med., 28, 692-728 (2007) (DOI: 10.1016/j.mam.2007.03.001).
- Traber, M.G. and Atkinson, J. Vitamin E, antioxidant and nothing more. Free Rad. Biol. Med., 43, 4-15 (2007) (DOI: 10.1016/j.freeradbiomed.2007.03.024).
- Nesaretnam, K., Yew, W.W. and Wahid, M.B. Tocotrienols and cancer, beyond antioxidant activity. Eur. J. Lipid Sci. Technol., 109, 445-452 (2007).
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Rad. Biol. Med., 49, 503-515 (2010) (DOI: 10.1016/j.freeradbiomed.2010.04.016).
- Seppanen, C.M., Song, Q. and Csallany, A.S. The antioxidant functions of tocopherol and tocotrienol homologues in oils, fats, and food systems. J. Am. Oil Chem. Soc., 87, 469-481 (2010) (DOI: 10.1007/s11746-009-1526-9).
- Ruperez, F. J., Martin, D., Herrera, E. and Barbas, C. Chromatographic analysis of α-tocopherol and related compounds in various matrices. J. Chromatogr. A, 935, 45-69 (2001).
- Fratianni, A., Caboni, M.F., Irano, M. and Panfili, G. A critical comparison between traditional methods and supercritical carbon dioxide extraction for the determination of tocochromanols in cereals. Eur. Food Res. Technol., 215, 353-358 (2002).
- Panfili, G., Fratianni, A., and Irano, M. Normal phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. J. Agric. Food Chem., 51, 3940-3944 (2003).
- Ryynänen, M., Lampi, A., Salo-Väänänen, P., Ollilainen, V. and Piironen, V. A small-scale sample preparation method with HPLC analysis for determination of tocopherols and tocotrienols in cereals. J. Food Comp. Anal., 17, 749-765 (2004).
- Leo, L., Rescio, L., Ciurlia, L. and Zacheo, G. Supercritical carbon dioxide extraction of oil and α-tocopherol from almond seeds. J. Sci. Food Agric., 85, 2167-2174 (2005) (DOI: 10.1002/jsfa.2244).
- Kramer, J.K.G., Blais, L., Fouchard, R.C., Melnyk, R.A. and Kallury, K.M.R. A rapid method for the determination of vitamin E forms in tissues and diet by high-performance liquid chromatography using a normal-phase diol column. Lipids, 32, 323-330 (1997).
- Franke, A.A., Murphy, S.P., Lacey, R. and Custer, L.J. Tocopherol and tocotrienol levels of foods consumed in Hawaii. J. Agric. Food Chem., 55, 769-778 (2007).
- Stevenson, D.G., Eller, F.J., Wang, L., Jane, J., Wang, T. and Inglett, G.E. Oil and tocopherol content and composition of pumpkin seed oil in 12 cultivars. J. Agric. Food Chem., 55, 4005-4013 (2007) (DOI: 10.1021/jf0706979).
- Peterson, D.M., Jensen, C.M., Hoffman, D.L. and Mannerstedt-Fogelfors, B. Oat tocols, Saponification vs. direct extraction and analysis in high-oil genotypes. Cereal Chem., 84, 56-60 (2007).
- Lee, B.L., New, A.L. and Ong, C.N. Simultaneous determination of tocotrienols, tocopherols, retinol, and major carotenoids in human plasma. Clin. Chem., 49, 2056-2066 (2003).
- Kadioglu, Y., Demirkaya, F. and Demirkaya, A.K. Quantitative determination of underivatized α-tocopherol in cow milk, vitamin and multivitamin drugs by GC-FID. Chromatographia, 70, 665-670 (2009) (DOI: 10.1365/s10337-009-1184-y).
- Gómez-Coronado, D.J.M., Ibañez, E., Rupérez, F.J. and Barbas, C. Tocopherol measurement in edible products of vegetable origin. J. Chromatogr. A, 1054, 227-233 (2004) (DOI: 10.1016/j.chroma.2004.08.072).
- Nielsen, M.M. and Hansen, A. Rapid high-performance liquid chromatography determination of tocopherols and tocotrienols in cereals. Cereal Chem., 85, 248-251 (2008).
- Schwartz, H., Ollilainen, V., Piironen, V. and Lampi, A. Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. J. Food Comp. Anal., 21, 152-161 (2008).
- Lanina, S.A., Toledo, P., Sampels, S., Kamal-Eldin, A. and Jastrebova, J.A. Comparison of reversed-phase liquid chromatography-mass spectrometry with electrospray and atmospheric pressure chemical ionization for analysis of dietary tocopherols. J. Chromatogr. A, 1157, 159-170 (2007) (DOI: 10.1016/j.chroma.2007.04.058).
- Diwakar, B.T., Dutta, P.K., Lokesh, B.R., and Naidu, K.A. Physicochemical properties of garden cress (Lepidium sativum L.) seed oil. J. Am. Oil Chem. Soc., 87, 539-548 (2010) (DOI: 10.1007/s11746-009-1523-z).
- Junsoo, L., Lin, Y., Landen, W.O. and Eitenmiller, R.R. Optimization of an extraction procedure for the quantification of vitamin E in tomato and broccoli using response surface methodology. J. Food Comp. Anal., 13, 45-57 (2000).
- Abidi, S.L. Tocol-derived minor constituents in selected plant seed oils. J. Am. Oil Chem. Soc., 80, 327-333 (2003).
- Bustamante-Rangel, M., Delgado-Zamarreñno, M.M., Sanchez-Perez, A. and Carabias-Martínez, R.. Determination of tocopherols and tocotrienols in cereals by pressurized liquid extraction-liquid chromatography-mass spectrometry. Anal. Chim. Acta, 587, 216-221 (2007).
- Delgado-Zamarreño, M., Bustamante-Rangel, M., Sierra-Manzano, S., Verdugo-Jara, M. and Carabias-Martínez, R. Simultaneous extraction of tocotrienols and tocopherols from cereals using pressurized liquid extraction prior to LC determination. J. Sep. Sci., 32, 1430-1436 (2009) (DOI: 10.1002/jssc.200800707).
- Perretti, G., Marconi, O., Montanari, L. and Fantozzi, P. Fat-soluble vitamin extraction by analytical supercritical carbon dioxide. J. Am. Oil Chem. Soc., 80, 629-633 (2003).
- Perretti, G., Marconi, O., Montanari, L. and Fantozzi, P. Rapid determination of total fats and fat-soluble vitamins in Parmigiano cheese and salami by SFE. LWT - Food Sci. Technol., 37, 87-92 (2004) (DOI: 10.1016/S0023-6438(03)00138-5).
- Moreau, R.A., Powell, M.J. and Singh, V. Pressurized liquid extraction of polar and nonpolar lipids in corn and oats with hexane, methylene chloride, isopropanol, and ethanol. J. Am. Oil Chem. Soc., 80, 1063-1067 (2003).
- Delgado-Zamarreño, M.M., Bustamante-Rangel, M., García-Jiménez, M., Sánchez-Pérez, A. and Carabias-Martínez, R. Off-line coupling of pressurized liquid extraction and LC/ED for the determination of retinyl acetate and tocopherols in infant formulas. Talanta, 70, 1094-1099 (2006) (DOI: 10.1016/j.talanta.2006.02.020).
- Grigoriadou, D., Androulaki, A., Psomiadou, E. and Tsimidou, M.Z. Solid phase extraction in the analysis of squalene and tocopherols in olive oil. Food Chem., 105, 675-680 (2007) (DOI: 10.1016/j.foodchem.2006.12.065).
- Mata-Granados, J.M., Quesada Gómez, J.M. and Luque de Castro, M.D. Fully automatic method for the determination of fat soluble vitamins and vitamin D metabolites in serum. Clin. Chim. Acta, 403, 126-130 (2009) (DOI: 10.1016/j.cca.2009.01.029).
- Puoci, F., Cirillo, G., Curcio, M., Iemma, F., Spizzirri, U.G. and Picci, N. Molecularly imprinted solid phase extraction for the selective HPLC determination of α-tocopherol in bay leaves. Anal. Chim. Acta, 593, 164-170 (2007) (DOI: 10.1016/j.aca.2007.04.053).
- Liu, Z., Kang, X. and Fang, F. Solid phase extraction with electrospun nanofibers for determination of retinol and α-tocopherol in plasma. Microchim. Acta, 168, 59-64 (2010) (DOI: 10.1007/s00604-009-0263-y).
- Kamal-Eldin, A., Gorgen, S., Pettersson, J. and Lampi, A.M. Normal-phase high-performance liquid chromatography of tocopherols and tocotrienols - comparison of different chromatographic columns. J. Chromatogr. A, 881, 217-227 (2000).
- Ng, M.H., Choo, Y.M., Ma, A.N., Chuah, C.H. and Hashim, M.A. Separation of vitamin E (tocopherol, tocotrienol, and tocomonoenol) in palm oil. Lipids, 39, 1031-1035 (2004).
- Butinar, B., Bucar-Miklavcic, M., Mariani, C. and Raspor, P. New vitamin E isomers (gamma-tocomonoenol and alpha-tocomonoenol) in seeds, roasted seeds and roasted seed oil from the slovenian pumpkin variety 'Slovenska golica'. Food Chem., 128, 505-512 (2011) (DOI: 10.1016/j.foodchem.2011.03.072).
- AOCS. A.O.C.S. official method ce 8-89 (reapproved 1997) determination of tocopherols and tocotrienols in vegetable oils and fats by HPLC. 5 pp. In: Official Methods and Recommended Practices of the American Oil Chemists' Society 5th edition. American Oil Chemists' Society, Champaign, IL (1997).
- EN 12822. European standard EN 12822, 2000, Foodstuffs - determination of vitamin E by high performance liquid chromatography - measurement of alpha-, beta-, gamma-, and delta-tocopherols. 16 pp. European Committee for Standardization, Brussels, Belgium (2000).
- Podda, M., Weber, C., Traber, M.G. and Packer, L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J. Lipid Res., 37, 893-901 (1996).
- Moltó-Puigmartí, C., Castellote, A.I. and Carmen López-Sabater, M. Ultra-high-pressure liquid chromatographic method for the analysis of tocopherols in human colostrum and milk. J. Chromatogr. A, 1216, 4388-4394 (2009) (DOI: 10.1016/j.chroma.2009.02.088).
- Nagata, Y., Nishio, T. and Kanazawa, H. Reaction monitoring of tocopherols with active nitrogen oxides by ultra high-speed liquid chromatography. J. Pharm. Biomed. Anal., 55, 241-246 (2011) (DOI: 10.1016/j.jpba.2010.12.036).
- Khan, A., Khan, M.I., Iqbal, Z., Shah, Y., Ahmad, L. and Watson, D. G. An optimized and validated RP-HPLC/UV detection method for simultaneous determination of all-trans-retinol (vitamin A) and α-tocopherol (vitamin E) in human serum: Comparison of different particulate reversed-phase HPLC columns. J. Chromatogr. B, 878, 2339-2347 (2010) (DOI: 10.1016/j.jchromb.2010.07.009).
- Paz San Andres, M., Otero, J. and Vera, S. High performance liquid chromatography method for the simultaneous determination of α-, γ- and δ-tocopherol in vegetable oils in presence of hexadecyltrimethylammonium bromide/n-propanol in mobile phase. Food Chem. 126, 1470-1474 (2011) (DOI: 10.1016/j.foodchem.2010.11.161).
- Gentili, A. and Caretti, F. Evaluation of a method based on liquid chromatography-diode array detector-tandem mass spectrometry for a rapid and comprehensive characterization of the fat-soluble vitamin and carotenoid profile of selected plant foods. J. Chromatogr. A, 1218, 684-697 (2011) (DOI: 10.1016/j.chroma.2010.12.001).
- Chen, H., Angiuli, M., Ferrari, C., Tombari, E., Salvetti, G. and Bramanti, E. Tocopherol speciation as first screening for the assessment of extra virgin olive oil quality by reversed-phase high-performance liquid chromatography/fluorescence detector. Food Chem., 125, 1423-1429 (2011) (DOI: 10.1016/j.foodchem.2010.10.026).
- Lampi, A., Nurmi, T., Ollilainen, V. and Piironen, V. Tocopherols and tocotrienols in wheat genotypes in the HEALTHGRAIN diversity screen. J. Agric. Food Chem., 56, 9716-9721 (2008) (DOI: 10.1021/jf801092a).
- Alves, R.C., Casal, S., and Oliveira, M.B.P.P. Determination of vitamin E in coffee beans by HPLC using a micro-extraction method. Food Sci. Technol. Int., 15, 57-63 (2009).
In This Section
- Solid-phase extraction columns in the analysis of lipids
- Preparation of Ester Derivatives of Fatty Acids for Chromatographic Analysis
- Preparation of Lipid Extracts Tissues
- The Chromatographic Resolution of Chiral Lipids
- Detectors for HPLC of Lipids with Special Reference to Evaporative Lght-Scattering Detection
- Why Doesn't Your Method Work When I Try It?
- Laboratory Accreditation in a Lipid Analysis Context
- What Column do I Need for Gas Chromatographic Analysis of Fatty Acids?
- Fatty Acid Analysis by HPLC
- Alternatives to Methyl Esters for GC Analysis of Fatty Acids
- A Practical Guide to the Analysis of Conjugated Linoleic Acid (CLA)
- Application of Infrared Spectroscopy to the Rapid Determination of Total Saturated, trans, Monounsaturated, and Polyunsaturated Fatty Acids
- The Use of Lithiated Adducts for Structural Analysis of Acylglycerols by Mass Spectrometry with Electrospray Ionization
- Identification of FAME Double Bond Location by Covalent Adduct Chemical Ionization (CACI) Tandem Mass Spectrometry
- The Use of Countercurrent Chromatography (CCC) in Lipid Analysis
- Gas Chromatographic Analysis of Plant Sterols
- Analysis of Tocopherols and Tocotrienols by HPLC
- Reversed-Phase HPLC of Triacylglycerols
- Structural Analysis of Triacylglycerols
- Thin-Layer Chromatography of Lipids
- High-temperature Gas Chromatography of Triacylglycerols
- Modification of an AOCS Official Method for Crude Oil Content in Distillers Grains and Other Agricultural Materials
- Lipidomics - A Personal View