A Practical Guide to the Analysis of Conjugated Linoleic Acid (CLA)
This paper was first published in INFORM, 12, 147-152 (2001), and it is reproduced by kind permission of the American Oil Chemists' Society.
Introduction
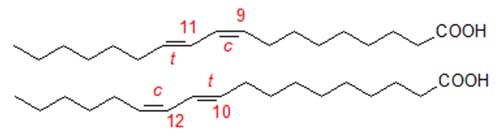
There is increasing interest in conjugated linoleic acid (CLA) because of its potential therapeutic properties. Natural CLA is known to consist of several geometrical isomers of which the most abundant is 9-cis,11-trans-octadecadienoic acid, formed by biohydrogenation in the rumen. Commercial CLA is produced by alkaline isomerization of linoleate-rich oils, such as sunflower oil, and tends to contain an equimolar mixture of 9-cis,11-trans- and 10-trans,12-cis-octadecadienoic acids, together with variable amounts (but up to 30%) of both geometrical and positional isomers.
In addition, these isomers can be elongated and desaturated in animal tissues by the enzymes involved in the biosynthesis of arachidonic acid to produce conjugated analogues, which may even be responsible for the biological activity of CLA [1]. In analysing CLA, it is therefore important that we be able to separate and quantify these geometrical and positional isomers, avoiding additional isomerization during any derivatization steps. Analytical methodology is especially important now that it is recognized that the various isomers may have very different effects in biological systems. We recommend especially the review by Banni and Martin [2] and the many chapters in a recent AOCS monograph [3]; these should be consulted for thorough coverage of the literature. This is a personal account of methods in use in the authors' laboratories with selected references only.
In considering the analysis of CLA, it is useful to treat the subject from two practical view points. Commercial CLA is usually supplied as the free acid with the components of interest being present at high levels, so analysis is relatively straightforward. In animal tissues, CLA is in esterified form and is present at low levels in general, so concentration steps may be required for characterization and analysis. However, some steps may be common to both aspects. Capillary gas chromatography (GC) with a column of the type used for the analysis of trans fatty acids, e.g. CP-Sil 88™ or BPX-70™ (100 m), will form the basis of the preferred approach, with GC-mass spectrometry (MS) as the 4,4-dimethyloxazoline (DMOX) derivatives and/or 4-methyl-1,2,4-triazoline-3,5-dione (MTAD) adducts being an invaluable adjunct. Silver ion high-performance liquid chromatography (HPLC) has proved very useful for the separation of geometrical and positional isomers. In addition, with natural samples, concentration of CLA isomers by reversed-phase HPLC and silver ion chromatography must be considered, prior to GC or GC-MS analysis.
Perhaps the single most comprehensive method for commercial CLA preparations has proved to be 13C-NMR spectroscopy [4], which permitted the identification and quantification of all the positional (7,9- to 11,13-18:2) and geometrical isomers (cis,trans-, trans,cis-, cis,cis- and trans,trans-) present in such samples. This is by far the most complete single analysis of CLA, but unfortunately the methodology requires substantial amounts of sample and is not likely to be applicable to tissue extracts at natural levels.
The following methods have been found satisfactory for derivatization and for the various chromatographic steps in the authors' laboratories. Others must consider the nature of their samples, how much information they require, what precision is necessary and how much time and effort they can devote to the analysis before deciding which approach to adopt.
Gas Chromatographic Analysis
Before fatty acids are analysed by GC, they must first be converted to the methyl esters. There is now a substantial body of work to confirm that acid-catalysed methylation is undesirable in general for the preparation of methyl esters, as it causes geometrical isomerization with an increase in the relative proportions of trans,trans isomers. However, there appear to be no significant drawbacks to the use of base-catalysed transesterification of lipids. Free fatty acids can be methylated on a small scale by means of a phase-transfer catalysed method. On the other hand, trimethylsilyl-diazomethane, which is often recommended, can produce artefacts (Claire Fernie, private communication). Alternatively, in spite of the caveat regarding acidic methylation, mild boron trifluoride- or sulfuric acid-methanol reactions can be employed provided that scrupulous attention is paid to detail.
Base-catalysed methylation is recognized to be best for esterified lipids as acid catalysis can cause isomerization of CLA. The following method is recommended.
| The lipid sample (up to 50 mg) is dissolved in dry toluene (1 mL) in a test tube, 0.5 M sodium methoxide in anhydrous methanol (2 mL) is added, and the solution is maintained at 50°C for 10 min. Glacial acetic acid (0.1 mL) is then added, followed by water (5 mL). The required esters are extracted into hexane (2 × 5 mL), using a Pasteur pipette to separate the layers. The hexane layer is dried over anhydrous sodium sulfate and filtered, before the solvent is removed under reduced pressure on a rotary film evaporator. The sample is dissolved in hexane (containing 50 ppm BHT) for GC analysis. |
A longer reaction time is necessary for cholesterol esters containing CLA. Of course, with samples such as milk fat that contain a high proportion of short-chain fatty acids, it is advisable to use modified methods to minimize the loss of butyric and hexanoic acids, especially [5].
Free acids are best methylated on a small scale by means of a phase-transfer catalysed method, or by a mild boron trifluoride-methanol reaction [6], although sulfuric acid-methanol (1%) can be used in a similar way with care (Claire Fernie, private communication).
| Phase transfer method: The sample (10 mg) is dissolved in dichloromethane (1 mL) in a culture tube (125 × 16 mm) with a PTFE-lined cap. To this is added 0.1M tetrabutylammonium hydrogen sulfate in 0.2M aqueous sodium hydroxide (1 mL) and methyl iodide (25 mL). After mixing and continuous shaking for 60 min, the layers are allowed to settle. The lower layer is taken by means of a Pasteur pipette, and the solvent is evaporated in a stream of nitrogen at 30°C. BF3-methanol: The free fatty acid (up to 10 mg) is reacted with BF3-methanol (1 mL; 14%) for 10 min at ambient temperature. The esters are extracted into hexane (2 mL) and washed by water (2 × 5 mL). The upper phase is dried over anhydrous sodium sulfate, then the solvent is evaporated without heating in a stream of nitrogen. |
Capillary columns of the Carbowax type are the standard in most laboratories for routine analysis of fatty acids, but they are of limited value for the analysis of CLA. The two main components, 9-cis,11-trans- and 10-trans,12-cis-18:2, are well separated from each other, but not from other positional isomers. The various types of geometrical isomers give distinct peaks, but within these groups, positional isomers are not fully resolved, unfortunately. For reasons that are not at all clear, with all polar phases, cis,trans- elute before cis,cis- before trans,trans- isomers. Even with these limitations, a single chromatographic run with almost any polar stationary phase will at least give an approximate figure for the total content of CLA relative to other components. It may be worth noting that lipid analysts appear to demand much higher standards of precision than is possible in most fields of biochemistry and nutrition.
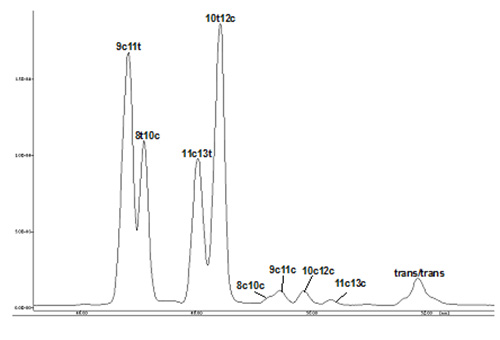
As mentioned above, long columns of the type favoured for analysis of trans-monoenoic fatty acids are required for resolution of CLA isomers, and good separations have been reported for CP-Sil 88™ and BPX-70™ columns, and an example of the latter is illustrated in Figure 1. CLA isomers elute long after nonconjugated dienes, and the important positional isomers emerge in the order -
9c,11t < 8t,10c < 11c,13t < 10t,12c
The authors have observed that it is not easy to obtain such separations reproducibly, and in particular the separation of the first two isomers is rarely easy.
Figure 1. GC separation of CLA methyl esters on a BPX70 column (120 m × 0.25 mm; 0.25 mm film thickness; SGE Ltd). The carrier gas was hydrogen (linear gas velocity at 180°C = 35.2 cm/s). Split-less injection was employed, and the column temperature was programmed: 60°C for 1 min, then increased at 20°C/min to 170°C and held at this temperature for a further 50 min.
There is little to confuse the analysis in commercial CLA samples, but when CLA is fed in nutritional experiments, other fatty acids may be present in tissues that confuse the picture. For example, 21:0 or 20:2 fatty acids may occur naturally and they elute in the same region of the chromatogram as the cis,cis- and trans,trans-isomers, especially. GC-MS may then be useful, both to to locate the double bonds and to identify any nonconjugated fatty acids that co-chromatograph with those of interest. Several types of nitrogen-containing derivatives have been developed for GC-MS in general, and of these DMOX derivatives have proved the most useful for conjugated dienes (reviewed by Spitzer in [3]). They are prepared by heating the fatty acid or lipid derivative with 2-amino-2-methylpropanol as follows -
| To the lipid sample (up to 2 mg) in a test tube is added 2-amino-2-methyl-1-propanol (0.25 g). The vessel is flushed with nitrogen, stoppered, and placed in a heating block, at 190°C overnight. On cooling, diethyl ether-hexane (1:1, v/v; 5 mL) is added to the tube, followed by water (5 mL). The organic layer is washed with distilled water (3 mL) and dried carefully over anhydrous sodium sulfate. The sample is taken to dryness in a gentle stream of nitrogen at 30°C, and is dissolved in a little hexane for GC-MS analysis. A few crystals of anhydrous sodium sulfate help to stabilize the derivatives. |
DMOX derivatives can be subjected to GC analysis under similar conditions to methyl esters, and they afford comparable resolution. However, in view of the harsh conditions required for preparation of the derivatives, we suggest that a rigorous test of the methodology with pure standards is required if they are to be used quantitatively.
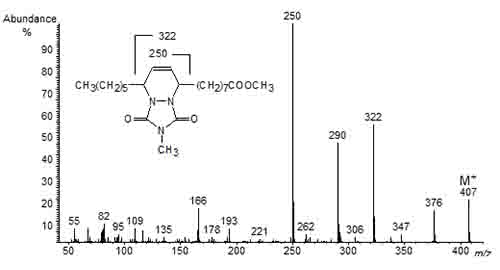
A valuable alternative consists in the use of a Diels-Alder reaction to prepare the 4-methyl-1,2,4-triazoline-3,5-dione (MTAD) adducts of the conjugated fatty acids. The reagent reacts almost instantaneously with the conjugated double bond system, and the adducts have excellent mass spectrometric properties that enable location of the conjugated double bonds, as illustrated in Figure 2 for the MTAD adduct of methyl 9,11-octadecadienoate [7]. The ions at m/z = 250 and 322 represent cleavage on either side of the six-membered ring that contains the carbons of the original conjugated double bond system.
Figure 2. Mass spectrum of the MTAD adduct of methyl 9,11-octadecadienoate.
The reaction is carried out as follows -
The CLA methyl ester (220 mg; 1.15 mM) and MTAD (425 mg; 5.8 mM) in dichloromethane (650 mL) are mixed in a test tube at 0°C by agitating for less than 10 seconds. The reaction is stopped immediately by addition of 1,3-hexadiene, followed by agitation for a few seconds. Excess reagents are removed a stream of nitrogen at 30°C, and the sample is redissolved in dichloromethane for analysis by GC-MS.
By means of GC-MS with selective ion monitoring, excellent results have been obtained with commercial CLA that complemented the NMR results [4]. On the other hand, with CLA at natural levels in tissues it is advisable to obtain a concentrate before applying the derivatization procedure, as we have sometimes observed some nospecific reaction with polyunsaturated fatty acids.
Not only have these methods been used to characterize conjugated dienes in CLA samples, but they have also been employed to prove the structures of CLA metabolites formed in tissues, such as 5,8,11,13- and 5,8,12,14-eicosatetraenoic acids [1].
Silver Ion Chromatography
In silver ion thin-layer chromatography, conjugated dienes tend to elute close to monoenes. Very much better resolution is possible by silver ion HPLC using columns packed with ion-exchange media loaded with silver ions. There have been three approaches to the problem, but these have not been compared objectively in the laboratory so it is not yet possible to state which is best.
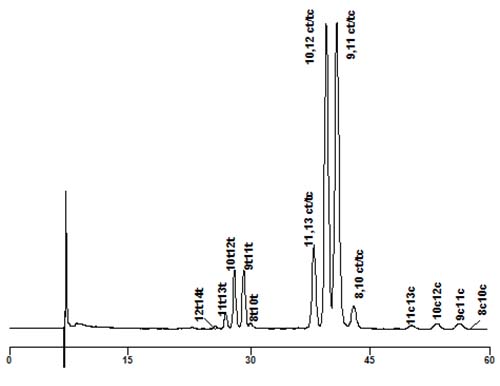
The first approach was pioneered by Adlof but then greatly improved by Yurawecz, Kramer and colleagues who reviewed the topic in [3]. They utilized a mobile phase of hexane containing a small amount of acetonitrile to separate the methyl ester derivatives, using the specific UV absorbance of the conjugated double bonds for detection and quantification. By coupling as many as six columns in series (though two were adequate for most practical purposes), some remarkable separations were achieved, both of positional and geometrical isomers. trans,trans-Isomers eluted first, followed by cis,trans- then cis,cis-, and within each group many positional isomers were clearly resolved. As an example, Figure 3 illustrates a separation of a commercial CLA sample. In this instance, each peak was identified by collecting it and converting to the MTAD adduct for GC-MS.
Figure 3. Silver ion chromatography of a commercial CLA mixture (Sigma-Aldrich Inc.). Two Chromspher Lipids™ columns (250 × 4.6 mm, i.d.; Varian Inc.) were used in series with a mobile phase of hexane-acetonitrile (99.9:0.1, v/v) at a flow rate of 1 mL/min, with UV detection at 234 nm.
The second approach consisted in the separation of CLA isomers as the p-methoxyphenacyl esters with dichloromethane-hexane-acetonitrile mixtures as mobile phase [8]. In this instance, only a single chromatographic column was required. As detection was by the absorbance of the p-methoxyphenacyl moiety at 270 nm, all fatty acids were detected and quantified, not simply the conjugated dienes.
Finally, good resolution of CLA as the free acids has recently been reported, with hexane-acetonitrile-acetic acid mixtures as the mobile phase with specific detection of the conjugated double bonds at 234 nm [9]. This procedure may be of special value for commercial CLA samples, supplied as the free acids, since no derivatization step is required.
Reversed-phase HPLC with Second Derivative UV Detection
Conjugated dienes exhibit a distinct UV absorbance in the region of 230-235 nm, while isolated double bonds absorb at 206 to 210 nm. However, the latter can interfere with the analysis of CLA isomers in tissues, since they tend to be present at such low levels. Banni and coworkers were able to overcome this problem by taking the differential of the first derivative spectrum, thus calculating a second derivative with two distinct peaks with minima at 234 and 242 nm. This gave a much more sensitive and accurate estimate of the conjugated diene content of fatty acids, since the Beer-Lambert law is unaffected by differentiation. When combined with reversed-phase HPLC it was possible to separate and quantify the metabolites of CLA as well as CLA per se [2]. The technique has also been used in series with mass spectrometry [6].
Concentration of Natural CLA Isomers for Further Analysis
In order to have sufficient material for analysis with tissue samples containing CLA at low levels, a pre-concentration step may be required. This can be accomplished either by reversed-phase HPLC or by silver ion chromatography or, better, by using the two techniques sequentially.
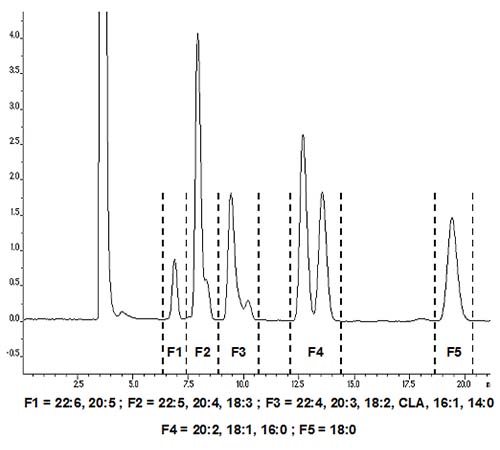
In reversed-phase chromatography, CLA isomers elute close to linoleate and can be collected preparatively by collecting a single broad C18 diene fraction. Many analysts add water to the mobile phase or use acetonitrile-water gradients. However, we prefer to use acetonitrile alone either at a constant flow rate or with a flow gradient, as this makes it easier to recover the required esters by evaporation of the mobile phase. Most columns of the octadecylsilyl (ODS) type can be used, but one of us employs base-stabilized Hichrom RPB™, as it can also be used with DMOX derivatives and picolinyl esters (but not free fatty acids) [10]. For methyl esters a more conventional ODS phase, such as Nucleosil C18™, is suitable [11]. Evaporative light-scattering detection with a stream splitter can be used, or UV detection at 206 nm (isolated double bonds) or 230 nm (conjugated double bonds), or refractive index detection. Keeping the column temperature constant aids reproducibility but is not essential. On a standard analytical column (4.6 mm diam.), about 1 mg of sample can be separated in micropreparative mode, but up to 20 mg can be chromatographed on a preparative column (10 mm diam.), as illustrated in Figure 4.
Figure 4. Reversed-phase separation of fatty acid methyl esters, including CLA. A Nucleosil C18 column (250 × 10 mm ID; 5 µm particles) was used with acetonitrile as mobile phase, and UV detection at 234 nm. Methyl esters (20 mg) in acetone were injected, with acetonitrile as mobile phase and a flow rate of 4 mL/min. The fraction corresponding to the C18 dienes may also contain some 14:0, 16:1 and certain polyunsaturated fatty acids.
Silver ion TLC and HPLC methods are available to obtain a concentrate of CLA, but a simple solid-phase extraction method adapted from a published procedure can be recommended [12]. It is conveniently applied to the C18 diene fraction isolated by reversed-phase HPLC as above.
| An Isolute™ SCX solid-phase extraction column (500 mg) or equivalent, wrapped to the level of the top of the adsorbent bed in aluminium foil, is preconditioned by elution with acetonitrile (2 mL). A solution of silver nitrate (20 mg) in acetonitrile-water (0.25 mL; 10:1, v/v) is then allowed to percolate through it. The column is flushed with acetonitrile (5 mL), acetone (5 mL) and dichloromethane (10 mL) by applying slight pressure from a pipette bulb, and is then ready for use. The methyl ester sample (0.1 to 0.25 mg) is applied to the column in dichloromethane (0.1 mL) and is washed onto the column with a similar volume of fresh solvent. Solvent mixtures are allowed to flow under gravity (approximately 0.5 mL/min). Saturated fatty acids are eluted with dichloromethane (5 mL), while the CLA-monoene fraction is eluted with dichloromethane-acetone (9:1, v/v; 5 mL). Linoleate remains on the column. Fractions are collected manually, and they can be analysed by GC after evaporation of the solvent. |
Acknowledgement: This work has been funded in part by the Scottish Executive Rural Affairs Department, and in part by the EU project No. FAIR 3671.
References
- Sébédio, J.L., Juanéda, P., Dobson, G., Ramilison, I., Martin, J.C., Chardigny, J.M. and Christie, W.W. Metabolites of conjugated isomers of linoleic acid (CLA) in the rat. Biochim. Biophys. Acta, 1345, 5-10 (1997).
- Banni, S. and Martin, J .-C. Conjugated linoleic acid and metabolites. In Trans Fatty Acids in Human Nutrition, pp. 261-302 (1998) (ed. J.L. Sébédio & W.W. Christie, Oily Press, Dundee).
- Yurawecz, M.P., Mossoba, M.M., Kramer, J.K.G., Pariza, M.W. and Nelson, G.J. Advances in Conjugated Linoleic Acid Research, Vol. 1. (1999) (AOCS Press, Champaign).
- Davis, A.L., McNeill, G.P. and Caswell, D.C. Analysis of conjugated linoleic acid isomers by 13C NMR spectroscopy. Chem. Phys. Lipids, 97, 155-165 (1999).
- Chouinard, P.Y., Corneau, L., Barbano, D.M., Metzger, L.E. and Bauman, D.E. Conjugated linoleic acids alter milk fatty acid composition and inhibit milk fat secretion in dairy cows. J. Nutr., 129, 1579-1584 (1999).
- Banni, S., Day, B.W., Evans, R.W., Corongui, F.P. and Lombardi, B. Liquid chromatographic-mass spectrometric analysis of conjugated diene fatty acids in a partially hydrogenated fat. J. Am. Oil Chem. Soc., 71, 1321-1325 (1994).
- Dobson, G. Identification of conjugated fatty acids by gas chromatography mass spectrometry of 4-methyl-1,2,4-triazoline-3,5-dione adducts. J. Am. Oil Chem. Soc., 75, 137-142 (1998).
- Nikolova-Damyanova, B., Momchilova, S. and Christie, W.W. Silver ion high-performance liquid chromatographic separation of conjugated linoleic acid isomers, and other fatty acids, after conversion to p-methoxyphenacyl derivatives. J. High Resolut. Chromatogr., 23, 348-352 (2000).
- Cross, R.F., Ostrowska, E., Muralitharan, H. and Dunshea, F.R. Mixed mode retention and the use of competing acid for the Ag+-HPLC analysis of underivatized conjugated linoleic acids. J. High Resolut. Chromatogr., 23, 317-323 (2000).
- Christie, W.W. Gas chromatography-mass spectrometry methods for structural analysis of fatty acids. Lipids, 33, 343-353 (1998).
- Juanéda, P. and Sébédio, J.L. Combined silver-ion and reversed-phase high-performance liquid chromatography for the separation and identification of C20 metabolites of conjugated linoleic acid isomers in rat liver lipids. J. Chromatogr. B, 724, 213-219 (1999).
- Christie, W.W. Silver ion chromatography using solid-phase extraction columns packed with a bonded-sulfonic acid phase. J. Lipid Res., 30, 1471-1473 (1989).
The lipid sample (up to 50 mg) is dissolved in dry toluene (1 mL) in a test tube, 0.5 M sodium methoxide in anhydrous methanol (2 mL) is added, and the solution is maintained at 50°C for 10 min. Glacial acetic acid (0.1 mL) is then added, followed by water (5 mL). The required esters are extracted into hexane (2 × 5 mL), using a Pasteur pipette to separate the layers. The hexane layer is dried over anhydrous sodium sulfate and filtered, before the solvent is removed under reduced pressure on a rotary film evaporator. The sample is dissolved in hexane (containing 50 ppm BHT) for GC analysis.
A longer reaction time is necessary for cholesterol esters containing CLA. Of course, with samples such as milk fat that contain a high proportion of short-chain fatty acids, it is advisable to use modified methods to minimize the loss of butyric and hexanoic acids, especially [5].
Free acids are best methylated on a small scale by means of a phase-transfer catalysed method, or by a mild boron trifluoride-methanol reaction [6], although sulfuric acid-methanol (1%) can be used in a similar way with care (Claire Fernie, private communication).
Phase transfer method: The sample (10 mg) is dissolved in dichloromethane (1 mL) in a culture tube (125 × 16 mm) with a PTFE-lined cap. To this is added 0.1M tetrabutylammonium hydrogen sulfate in 0.2M aqueous sodium hydroxide (1 mL) and methyl iodide (25 mL). After mixing and continuous shaking for 60 min, the layers are allowed to settle. The lower layer is taken by means of a Pasteur pipette, and the solvent is evaporated in a stream of nitrogen at 30°C.
BF3-methanol: The free fatty acid (up to 10 mg) is reacted with BF3-methanol (1 mL; 14%) for 10 min at ambient temperature. The esters are extracted into hexane (2 mL) and washed by water (2 × 5 mL). The upper phase is dried over anhydrous sodium sulfate, then the solvent is evaporated without heating in a stream of nitrogen.
Capillary columns of the Carbowax type are the standard in most laboratories for routine analysis of fatty acids, but they are of limited value for the analysis of CLA. The two main components, 9-cis,11-trans- and 10-trans,12-cis-18:2, are well separated from each other, but not from other positional isomers. The various types of geometrical isomers give distinct peaks, but within these groups, positional isomers are not fully resolved, unfortunately. For reasons that are not at all clear, with all polar phases, cis,trans- elute before cis,cis- before trans,trans- isomers. Even with these limitations, a single chromatographic run with almost any polar stationary phase will at least give an approximate figure for the total content of CLA relative to other components. It may be worth noting that lipid analysts appear to demand much higher standards of precision than is possible in most fields of biochemistry and nutrition.
As mentioned above, long columns of the type favoured for analysis of trans-monoenoic fatty acids are required for resolution of CLA isomers, and good separations have been reported for CP-Sil 88™ and BPX-70™ columns, and an example of the latter is illustrated in Figure 1. CLA isomers elute long after nonconjugated dienes, and the important positional isomers emerge in the order -
9c,11t < 8t,10c < 11c,13t < 10t,12c
The authors have observed that it is not easy to obtain such separations reproducibly, and in particular the separation of the first two isomers is rarely easy.
Figure 1. GC separation of CLA methyl esters on a BPX70 column (120 m × 0.25 mm; 0.25 mm film thickness; SGE Ltd). The carrier gas was hydrogen (linear gas velocity at 180°C = 35.2 cm/s). Split-less injection was employed, and the column temperature was programmed: 60°C for 1 min, then increased at 20°C/min to 170°C and held at this temperature for a further 50 min.
There is little to confuse the analysis in commercial CLA samples, but when CLA is fed in nutritional experiments, other fatty acids may be present in tissues that confuse the picture. For example, 21:0 or 20:2 fatty acids may occur naturally and they elute in the same region of the chromatogram as the cis,cis- and trans,trans-isomers, especially. GC-MS may then be useful, both to to locate the double bonds and to identify any nonconjugated fatty acids that co-chromatograph with those of interest. Several types of nitrogen-containing derivatives have been developed for GC-MS in general, and of these DMOX derivatives have proved the most useful for conjugated dienes (reviewed by Spitzer in [3]). They are prepared by heating the fatty acid or lipid derivative with 2-amino-2-methylpropanol as follows -
To the lipid sample (up to 2 mg) in a test tube is added 2-amino-2-methyl-1-propanol (0.25 g). The vessel is flushed with nitrogen, stoppered, and placed in a heating block, at 190°C overnight. On cooling, diethyl ether-hexane (1:1, v/v; 5 mL) is added to the tube, followed by water (5 mL). The organic layer is washed with distilled water (3 mL) and dried carefully over anhydrous sodium sulfate. The sample is taken to dryness in a gentle stream of nitrogen at 30°C, and is dissolved in a little hexane for GC-MS analysis. A few crystals of anhydrous sodium sulfate help to stabilize the derivatives.
DMOX derivatives can be subjected to GC analysis under similar conditions to methyl esters, and they afford comparable resolution. However, in view of the harsh conditions required for preparation of the derivatives, we suggest that a rigorous test of the methodology with pure standards is required if they are to be used quantitatively.
A valuable alternative consists in the use of a Diels-Alder reaction to prepare the 4-methyl-1,2,4-triazoline-3,5-dione (MTAD) adducts of the conjugated fatty acids. The reagent reacts almost instantaneously with the conjugated double bond system, and the adducts have excellent mass spectrometric properties that enable location of the conjugated double bonds, as illustrated in Figure 2 for the MTAD adduct of methyl 9,11-octadecadienoate [7]. The ions at m/z = 250 and 322 represent cleavage on either side of the six-membered ring that contains the carbons of the original conjugated double bond system.
Figure 2. Mass spectrum of the MTAD adduct of methyl 9,11-octadecadienoate.
The reaction is carried out as follows -
The CLA methyl ester (220 mg; 1.15 mM) and MTAD (425 mg; 5.8 mM) in dichloromethane (650 mL) are mixed in a test tube at 0°C by agitating for less than 10 seconds. The reaction is stopped immediately by addition of 1,3-hexadiene, followed by agitation for a few seconds. Excess reagents are removed a stream of nitrogen at 30°C, and the sample is redissolved in dichloromethane for analysis by GC-MS.
By means of GC-MS with selective ion monitoring, excellent results have been obtained with commercial CLA that complemented the NMR results [4]. On the other hand, with CLA at natural levels in tissues it is advisable to obtain a concentrate before applying the derivatization procedure, as we have sometimes observed some nospecific reaction with polyunsaturated fatty acids.
Not only have these methods been used to characterize conjugated dienes in CLA samples, but they have also been employed to prove the structures of CLA metabolites formed in tissues, such as 5,8,11,13- and 5,8,12,14-eicosatetraenoic acids [1].
Silver Ion Chromatography
In silver ion thin-layer chromatography, conjugated dienes tend to elute close to monoenes. Very much better resolution is possible by silver ion HPLC using columns packed with ion-exchange media loaded with silver ions. There have been three approaches to the problem, but these have not been compared objectively in the laboratory so it is not yet possible to state which is best.
The first approach was pioneered by Adlof but then greatly improved by Yurawecz, Kramer and colleagues who reviewed the topic in [3]. They utilized a mobile phase of hexane containing a small amount of acetonitrile to separate the methyl ester derivatives, using the specific UV absorbance of the conjugated double bonds for detection and quantification. By coupling as many as six columns in series (though two were adequate for most practical purposes), some remarkable separations were achieved, both of positional and geometrical isomers. trans,trans-Isomers eluted first, followed by cis,trans- then cis,cis-, and within each group many positional isomers were clearly resolved. As an example, Figure 3 illustrates a separation of a commercial CLA sample. In this instance, each peak was identified by collecting it and converting to the MTAD adduct for GC-MS.
Figure 3. Silver ion chromatography of a commercial CLA mixture (Sigma-Aldrich Inc.). Two Chromspher Lipids™ columns (250 × 4.6 mm, i.d.; Varian Inc.) were used in series with a mobile phase of hexane-acetonitrile (99.9:0.1, v/v) at a flow rate of 1 mL/min, with UV detection at 234 nm.
The second approach consisted in the separation of CLA isomers as the p-methoxyphenacyl esters with dichloromethane-hexane-acetonitrile mixtures as mobile phase [8]. In this instance, only a single chromatographic column was required. As detection was by the absorbance of the p-methoxyphenacyl moiety at 270 nm, all fatty acids were detected and quantified, not simply the conjugated dienes.
Finally, good resolution of CLA as the free acids has recently been reported, with hexane-acetonitrile-acetic acid mixtures as the mobile phase with specific detection of the conjugated double bonds at 234 nm [9]. This procedure may be of special value for commercial CLA samples, supplied as the free acids, since no derivatization step is required.
Reversed-phase HPLC with Second Derivative UV Detection
Conjugated dienes exhibit a distinct UV absorbance in the region of 230-235 nm, while isolated double bonds absorb at 206 to 210 nm. However, the latter can interfere with the analysis of CLA isomers in tissues, since they tend to be present at such low levels. Banni and coworkers were able to overcome this problem by taking the differential of the first derivative spectrum, thus calculating a second derivative with two distinct peaks with minima at 234 and 242 nm. This gave a much more sensitive and accurate estimate of the conjugated diene content of fatty acids, since the Beer-Lambert law is unaffected by differentiation. When combined with reversed-phase HPLC it was possible to separate and quantify the metabolites of CLA as well as CLA per se [2]. The technique has also been used in series with mass spectrometry [6].
Concentration of Natural CLA Isomers for Further Analysis
In order to have sufficient material for analysis with tissue samples containing CLA at low levels, a pre-concentration step may be required. This can be accomplished either by reversed-phase HPLC or by silver ion chromatography or, better, by using the two techniques sequentially.
In reversed-phase chromatography, CLA isomers elute close to linoleate and can be collected preparatively by collecting a single broad C18 diene fraction. Many analysts add water to the mobile phase or use acetonitrile-water gradients. However, we prefer to use acetonitrile alone either at a constant flow rate or with a flow gradient, as this makes it easier to recover the required esters by evaporation of the mobile phase. Most columns of the octadecylsilyl (ODS) type can be used, but one of us employs base-stabilized Hichrom RPB™, as it can also be used with DMOX derivatives and picolinyl esters (but not free fatty acids) [10]. For methyl esters a more conventional ODS phase, such as Nucleosil C18™, is suitable [11]. Evaporative light-scattering detection with a stream splitter can be used, or UV detection at 206 nm (isolated double bonds) or 230 nm (conjugated double bonds), or refractive index detection. Keeping the column temperature constant aids reproducibility but is not essential. On a standard analytical column (4.6 mm diam.), about 1 mg of sample can be separated in micropreparative mode, but up to 20 mg can be chromatographed on a preparative column (10 mm diam.), as illustrated in Figure 4.
Figure 4. Reversed-phase separation of fatty acid methyl esters, including CLA. A Nucleosil C18 column (250 × 10 mm ID; 5 µm particles) was used with acetonitrile as mobile phase, and UV detection at 234 nm. Methyl esters (20 mg) in acetone were injected, with acetonitrile as mobile phase and a flow rate of 4 mL/min. The fraction corresponding to the C18 dienes may also contain some 14:0, 16:1 and certain polyunsaturated fatty acids.
Silver ion TLC and HPLC methods are available to obtain a concentrate of CLA, but a simple solid-phase extraction method adapted from a published procedure can be recommended [12]. It is conveniently applied to the C18 diene fraction isolated by reversed-phase HPLC as above.
An IsoluteTM SCX solid-phase extraction column (500 mg) or equivalent, wrapped to the level of the top of the adsorbent bed in aluminium foil, is preconditioned by elution with acetonitrile (2 mL). A solution of silver nitrate (20 mg) in acetonitrile-water (0.25 mL; 10:1, v/v) is then allowed to percolate through it. The column is flushed with acetonitrile (5 mL), acetone (5 mL) and dichloromethane (10 mL) by applying slight pressure from a pipette bulb, and is then ready for use. The methyl ester sample (0.1 to 0.25 mg) is applied to the column in dichloromethane (0.1 mL) and is washed onto the column with a similar volume of fresh solvent. Solvent mixtures are allowed to flow under gravity (approximately 0.5 mL/min). Saturated fatty acids are eluted with dichloromethane (5 mL), while the CLA-monoene fraction is eluted with dichloromethane-acetone (9:1, v/v; 5 mL). Linoleate remains on the column. Fractions are collected manually, and they can be analysed by GC after evaporation of the solvent.
Acknowledgement: This work has been funded in part by the Scottish Executive Rural Affairs Department, and in part by the EU project No. FAIR 3671.
References
- Sébédio, J.L., Juanéda, P., Dobson, G., Ramilison, I., Martin, J.C., Chardigny, J.M. and Christie, W.W. Metabolites of conjugated isomers of linoleic acid (CLA) in the rat. Biochim. Biophys. Acta, 1345, 5-10 (1997).
- Banni, S. and Martin, J .-C. Conjugated linoleic acid and metabolites. In Trans Fatty Acids in Human Nutrition, pp. 261-302 (1998) (ed. J.L. Sébédio & W.W. Christie, Oily Press, Dundee).
- Yurawecz, M.P., Mossoba, M.M., Kramer, J.K.G., Pariza, M.W. and Nelson, G.J. Advances in Conjugated Linoleic Acid Research, Vol. 1. (1999) (AOCS Press, Champaign).
- Davis, A.L., McNeill, G.P. and Caswell, D.C. Analysis of conjugated linoleic acid isomers by 13C NMR spectroscopy. Chem. Phys. Lipids, 97, 155-165 (1999).
- Chouinard, P.Y., Corneau, L., Barbano, D.M., Metzger, L.E. and Bauman, D.E. Conjugated linoleic acids alter milk fatty acid composition and inhibit milk fat secretion in dairy cows. J. Nutr., 129, 1579-1584 (1999).
- Banni, S., Day, B.W., Evans, R.W., Corongui, F.P. and Lombardi, B. Liquid chromatographic-mass spectrometric analysis of conjugated diene fatty acids in a partially hydrogenated fat. J. Am. Oil Chem. Soc., 71, 1321-1325 (1994).
- Dobson, G. Identification of conjugated fatty acids by gas chromatography mass spectrometry of 4-methyl-1,2,4-triazoline-3,5-dione adducts. J. Am. Oil Chem. Soc., 75, 137-142 (1998).
- Nikolova-Damyanova, B., Momchilova, S. and Christie, W.W. Silver ion high-performance liquid chromatographic separation of conjugated linoleic acid isomers, and other fatty acids, after conversion to p-methoxyphenacyl derivatives. J. High Resolut. Chromatogr., 23, 348-352 (2000).
- Cross, R.F., Ostrowska, E., Muralitharan, H. and Dunshea, F.R. Mixed mode retention and the use of competing acid for the Ag+-HPLC analysis of underivatized conjugated linoleic acids. J. High Resolut. Chromatogr., 23, 317-323 (2000).
- Christie, W.W. Gas chromatography-mass spectrometry methods for structural analysis of fatty acids. Lipids, 33, 343-353 (1998).
- Juanéda, P. and Sébédio, J.L. Combined silver-ion and reversed-phase high-performance liquid chromatography for the separation and identification of C20 metabolites of conjugated linoleic acid isomers in rat liver lipids. J. Chromatogr. B, 724, 213-219 (1999).
- Christie, W.W. Silver ion chromatography using solid-phase extraction columns packed with a bonded-sulfonic acid phase. J. Lipid Res., 30, 1471-1473 (1989).
In This Section
- Solid-phase extraction columns in the analysis of lipids
- Preparation of Ester Derivatives of Fatty Acids for Chromatographic Analysis
- Preparation of Lipid Extracts Tissues
- The Chromatographic Resolution of Chiral Lipids
- Detectors for HPLC of Lipids with Special Reference to Evaporative Lght-Scattering Detection
- Why Doesn't Your Method Work When I Try It?
- Laboratory Accreditation in a Lipid Analysis Context
- What Column do I Need for Gas Chromatographic Analysis of Fatty Acids?
- Fatty Acid Analysis by HPLC
- Alternatives to Methyl Esters for GC Analysis of Fatty Acids
- A Practical Guide to the Analysis of Conjugated Linoleic Acid (CLA)
- Application of Infrared Spectroscopy to the Rapid Determination of Total Saturated, trans, Monounsaturated, and Polyunsaturated Fatty Acids
- The Use of Lithiated Adducts for Structural Analysis of Acylglycerols by Mass Spectrometry with Electrospray Ionization
- Identification of FAME Double Bond Location by Covalent Adduct Chemical Ionization (CACI) Tandem Mass Spectrometry
- The Use of Countercurrent Chromatography (CCC) in Lipid Analysis
- Gas Chromatographic Analysis of Plant Sterols
- Analysis of Tocopherols and Tocotrienols by HPLC
- Reversed-Phase HPLC of Triacylglycerols
- Structural Analysis of Triacylglycerols
- Thin-Layer Chromatography of Lipids
- High-temperature Gas Chromatography of Triacylglycerols
- Modification of an AOCS Official Method for Crude Oil Content in Distillers Grains and Other Agricultural Materials
- Lipidomics - A Personal View