Quantification by 1H-NMR
The Author: Gerhard Knothe, National Center for Agricultural Utilization Research, Agricultural Research Service, U.S. Department of Agriculture, Peoria, IL, USA.
1H-NMR is useful for a variety of quantitative analytical purposes in the chemistry of fats and oils that are briefly discussed here.
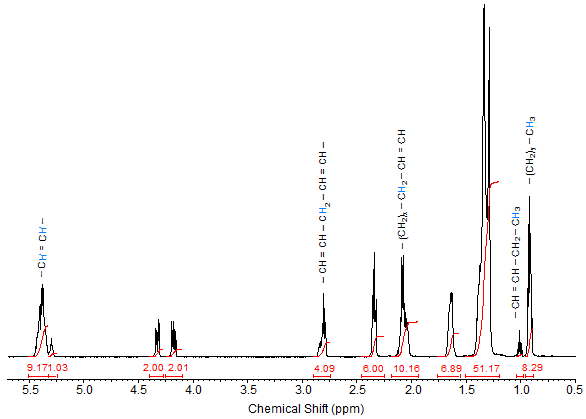
Fatty Acid Profile. The fatty acid profile of fats and oils as well as their derivatives such as alkyl esters is the major factor influencing their chemical and physical properties and subsequently their various applications. While GC is usually the method of choice for determining the fatty acid profile, the common unsaturated fatty acids (oleic, linoleic, linolenic) in an oil or fat can be quantified using 1H-NMR. This method utilizes the area per proton (determined by integration) and gives equations for determining the amounts of the unsaturated fatty acids. The results agree well with GC conducted as a control, although reportedly 13C-NMR results correlate even better with GC (Miyake et al., 1998). As an example, the 1H-NMR spectrum of soybean oil is given in Figure 1.
Figure 1. 1H-NMR spectrum of soybean oil.
Initially, the integration values of the protons need to be divided by 3. Using the integration values given in Figure 1 (integration values shown only for the relevant peaks; signal of the C2 protons in the chain used as reference; integration of terminal methyl in 18:3 = 0.81) and the equations (Knothe and Kenar, 2004) -
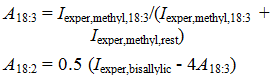
in which 'A' indicates the amounts of the subscripted fatty acids and 'I' indicates the experimentally determined integration values of the terminal methyl, bis-allylic and allylic protons, the fatty acid composition of soybean oil can be determined. The result is 18:1 = 25.40%, 18:2 = 50.36%, 18:3 = 8.90% with the rest (15.33%) being accounted for by saturates is obtained. These values agree well with the known average fatty acid composition of soybean oil. Related work is Miyake et al. (1998a).
Docosahexaenoic acid and ω-3 fatty acids in fish oils can be determined by 1H-NMR in good agreement with GC analyses (Igarashi et al., 2000). Other authors also reported on the determination of ω-3 fatty acids in fish oils (Aursand et al., 1993, Sacchi et al., 1993) Related, although less specific, parameters such as the iodine value, which reflects the total amount of unsaturated fatty acids in an oil or fat, can also be determined by 1H-NMR (Miyake et al., 1998b). The key to the determination is that the signal of the terminal methyl group in ω-3 fatty acids is shifted slightly downfield from that of fatty acids without this double bond (see also the webpage on unsaturated fatty acids). The triacylglycerols in whole vegetable seeds were determined quantitatively using a magic angle spinning technique (Wollenberg, 1991).
Identification verification of vegetable oils. While different oils or fats may be deliberately mixed for specific reasons, the adulteration of high-value oils with oils of lesser value constitutes a problem of economic and commercial significance when the adulterated oil is marketed as the pure, high-value oil. This is a major problem for olive oil, which is an expensive oil of recognized nutritional value. Accordingly, numerous research papers from major olive oil-producing Mediterranean countries, such as Greece, Italy and Spain, deal with identifying lower-value oils, such as hazelnut oil, used for adulterating olive oil. The adulteration problem is complicated by the fact that the lower-value oils usually have fatty acid profiles similar to olive oil. Among the methods used for analyzing potentially adulterated olive oil is 1H-NMR (besides 13C-NMR). For example, NMR was utilized in a study applying multivariate statistical methods to certain peaks of olive oil diluted with hazelnut or sunflower oil (Fauhl et al., 2000). Besides analyzing olive oil for diluents, 1H-NMR, together with analytical data, can be used in assessing the variety and geographical origin of the oil (Sacco et al., 2000). Even when not quantifying any components, the peak differences in NMR can be used to distinguish vegetable oils by visual inspection of the spectra (Guillén and Ruiz, 2003). Overviews of methods for assessing the quality and adulteration status of olive oil are available (Sacchi et al., 1997, Mannina and Segre, 2002).
Monitoring of oxidation. The oxidation of vegetable oils or their derivatives is an important quality problem and can lead to further deterioration of the oil. Especially the more unsaturated fatty acids with bis-allylic methylene groups are susceptible to oxidation. In studies of oxidation by 1H-NMR, primary oxidation products such as hydroperoxides and secondary oxidation products such as aldehydes were detected (Guillén and Ruiz, 2001, Guillén and Ruiz, 2005). Oxidation of ethyl docosahexaenoate was also determined by 1H-NMR and correlated with traditional methods (Falch et al., 2004). 1H-NMR is especially useful for such studies since for this method the samples do not require any further treatment which could cause changes to the samples themselves.
Reaction monitoring (mixtures of vegetable oils with other fatty compounds). 1H-NMR has been used for reaction monitoring, which actually constitutes analysing mixtures of vegetable oils with other fatty compounds. An example is the transesterification reaction of a vegetable oil to its corresponding methyl esters (Knothe, 2001a), a reaction that is steadily gaining significance due to the increasing production and use of biodiesel. In this case, the strong singlet peak of the resulting methyl esters is useful in quantification. Methyl esters of vegetable oils such as soybean (in the United States), rapeseed (in Europe), palm oil (in countries with tropical climate), animal fats or even waste vegetable oils are the most common form of biodiesel.
Solid fat content (SFC). While the other methods discussed here usually rely on high-resolution, research-grade NMR instruments, a bench-top, low-resolution pulsed NMR instrument can be used for determining the SFC of an oil or fat. The method determines the amount of solid triacylglycerols in the oil or fat at different temperatures, with only the pulsed NMR signal of the liquid fat being measured (van Duynhoven et al., 1999). The low-temperature signal is proportional to the total liquid at 60°C. The method is used for quality control purposes in hydrogenation, blending and interesterification. The SFC is used to assess properties of food products such as hardness and mouth feel. Crystallization mechanisms of fat blends can be studied by kinetic SFC measurements. The NMR-based SFC method is considered to be more accurate than the older dilatometric method giving the solid fat index (SFI) as shown by an interlaboratory collaborative study. The results of SFC cannot be directly compared to the dilatometric SFI. The dilatometric procedure is also more labor-intensive and cumbersome than SFC determination by NMR.
Non-fatty (extraneous) materials. Two examples for the analysis of non-fatty or extraneous materials in fatty compounds by 1H-NMR illustrate this application. The first example is the quantification of other lipidic materials, such as sterols, in vegetable oils (Sacco et al., 2000). The signal of a methyl group at C-18 of sterols is reportedly especially useful for this purpose. The second example is the blends of biodiesel with conventional petroleum-derived diesel fuel. 1H-NMR may be applied to verifying the blend level of biodiesel with the petroleum-derived fuel (Knothe, 2001b), in which case again the strong peak of the methyl ester moiety is useful.
Literature:
- Aursand, M., Rainuzzo, J.R. and Grasdalen, H. Quantitative high-resolution 13C and 1H nuclear magnetic resonance of ω3 fatty acids from white muscle of atlantic salmon (Salmo salar). J. Am. Oil Chem. Soc., 70, 971-981 (1993).
- Falch, E., Anthonsen, H.W., Axelson, D.E. and Aursand, M. Correlation between 1H and traditional methods for determining lipid oxidation of ethyl docosahexaenoate. J. Am. Oil Chem. Soc., 81, 1105-1110 (2004).
- Fauhl, C., Reniero, F. and Guillou, C. 1H NMR as a tool for the analysis of mixtures of virgin olive oil with oils of different botanical origin. Magn. Reson. Chem., 38, 436-443 (2000).
- Guillén, M.D. and Ruiz, A. High resolution 1H nuclear magnetic resonance in the study of ed
- Guillén, M.D. and Ruiz, A. Edible oils: discrimination by 1H nuclear magnetic resonance. J. Sci. Food Agric., 83, 338-346 (2003).
- Guillén, M.D. and Ruiz, A. Monitoring the oxidation of unsaturated oils and formation of oxygenated aldehydes by proton NMR. Eur. J. Lipid Sci. Technol., 107, 36-47 (2005).
- Igarashi, T., Aursand, M., Hirata, Y., Gribbestad, I.S., Wada, S. and Nonaka, M. Nondestructive quantitative determination of docosahexaenoic acid and n-3 fatty acids in fish oils by high-resolution 1H nuclear magnetic resonance spectroscopy. J. Am. Oil Chem. Soc.,, 77, 737-748 (2000).
- Knothe, G. Monitoring a progressing transesterification reaction by fiber-optic near-infrared spectroscopy with correlation to 1H nuclear magnetic resonance spectroscopy. J. Am. Oil Chem. Soc., 77, 489-493 (2001a).
- Knothe, G. Determining the blend level of mixtures of biodiesel with conventional diesel fuel by fiber-optic near-infrared spectroscopy and 1H nuclear magnetic resonance spectroscopy. J. Am. Oil Chem. Soc., 78, 1025-1028 (2001b).
- Knothe, G. and Kenar, J.A. Determination of the fatty acid profile by 1H NMR spectroscopy. Eur. J. Lipid Sci. Technol., 106, 88-96 (2004).
- Mannina, L. and Segre, A. High resolution nuclear magnetic resonance: from chemical structure to food authenticity. Grasas y Aceites, 53, 22-33 (2002).
- Miyake, Y. Yokomizo, K. and Matsuzaki, N. Determination of unsaturated fatty acid composition by high-resolution nuclear magnetic resonance spectroscopy. J. Am. Oil Chem. Soc., 75, 1091-1094 (1998a).
- Miyake, Y. Yokomizo, K. and Matsuzaki, N. Rapid determination of iodine value by 1H nuclear magnetic resonance spectroscopy. J. Am. Oil Chem. Soc., 75, 15-19 (1998b).
- Sacchi, R., Addeo, F. and Paolillo, L. 1H and 13C NMR of virgin olive oil. An overview. Magn. Reson. Chem., 35, S133-S145 (1997).
- Sacchi, R., Medina, I., Aubourg, S.P., Addeo, F. and Paolillo, L. Proton nuclear magnetic resonance rapid and structure-specific determination of ω-3 polyunsaturated fatty acids in fish lipids. J. Am. Oil Chem. Soc., 70, 225-228 (1993).
- Sacco, A., Brescia, M.A., Liuzzi, V., Reniero, F., Guillou, C., Ghelli, S. and van der Meer, P. Characterization of Italian olive oils based on analytical and nuclear magnetic resonance determinations. J. Am. Oil Chem. Soc., 77, 619-625 (2000).
- van Duynhoven, J., Goudappel, G.J., Gribnau, M.C.M. and Shukla, V.K.S. Solid fat content determination by NMR. INFORM, 10, 479-484 (1999).
- Wollenberg, K. Quantitative triacylglycerol analysis of whole vegetable seeds by 1H and 13C magic angle sample spinning NMR spectroscopy. J. Am. Oil Chem. Soc., 68, 391-400 (1991).
In This Section
- Introduction of NMR
- Saturated Fatty Acids and Methyl Esters
- Alkyl Esters Other than Methyl
- Glycerol Esters
- Non-Conjugated Double Bonds
- Conjugated Linoleic Acid (CLA)
- Acetylenic Fatty Acids and Derivatives
- Branched-Chain and Cyclic Fatty Acids
- Epoxy Fatty Acids
- Hydroxy and Hydroperoxy Fatty Acids
- Oxo Fatty Acids
- Fatty Alcohols
- Some Miscellaneous Fatty Acids
- Quantification by 1H-NMR
- The NMR Spectrum
- Alkanoic Acids
- Monoenoic Acids
- Polyunsaturated Fatty Acids
- Non-Methylene-Interrupted Polyenoic Fatty Acids
- Acids with conjugated unsaturation
- Acetylenic and Allenic Acids and Esters
- Branched-Chain and Cyclic Fatty Acids
- Cyclic Fatty Acids
- Epoxides and Acyclic Ethers
- Hydroxy and Hydroperoxy Acids
- Oxo (Keto) Acids
- Acids, Esters (Alkyl, Glycerol, Waxes), Alcohols and Acetates, Amides, and Nitriles
- Esters of Glycerol and Other Polyhydric Alcohols
- Oils and Fats
- Regiospecific Analysis of Triacylglycerols