Solvent effects (pyridine, quinoline, and carbon tetrachloride studied) on the NMR spectra of saturated fatty acid methyl esters were discussed (Tulloch, 1966). Chemical shifts in carbon tetrachloride (CCl4) are given in Table 1.
| Table 1. Chemical shifts of hydroxy stearates (Tulloch, 1966) in CCl4 | ||||||
| OH position | —CH2—COOMe | —CHOH— | CH3— | |||
|---|---|---|---|---|---|---|
| 2 | – | 3.93 | 0.86 | |||
| 3 | 2.24, 2.28, 2.34 | 3.80 | 0.86 | |||
| 4 | 2.33 | 3.48 | 0.86 | |||
| 5 | 2.22 | 3.40 | 0.85 | |||
| 6 | 2.20 | 3.42 | 0.86 | |||
| 7 | 2.19 | 3.40 | 0.85 | |||
| 8 | 2.18 | 3.40 | 0.85 | |||
| 9 | 2.18 | 3.40 | 0.85 | |||
| 10 | 2.16 | 3.38 | 0.85 | |||
| 11 | 2.17 | 3.39 | 0.86 | |||
| 12 | 2.18 | 3.40 | 0.86 | |||
| 13 | 2.17 | 3.40 | 0.87 | |||
| 14 | 2.17 | 3.40 | 0.87 | |||
| 15 | 2.17 | 3.42 | 0.88 | |||
| 16 | 2.17 | 3.34 | 0.88 | |||
| 17 | 2.17 | 3.58 | 1.07 | |||
| 18 | 2.17 | 3.46 | – | |||
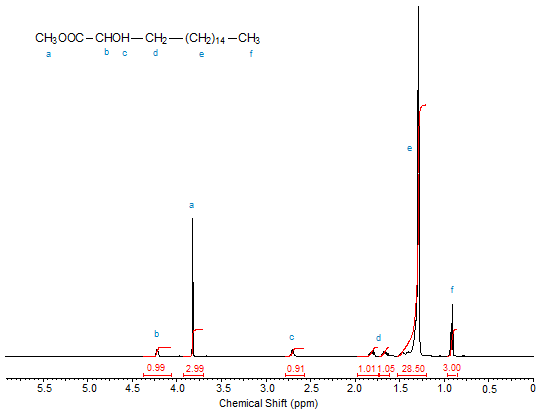
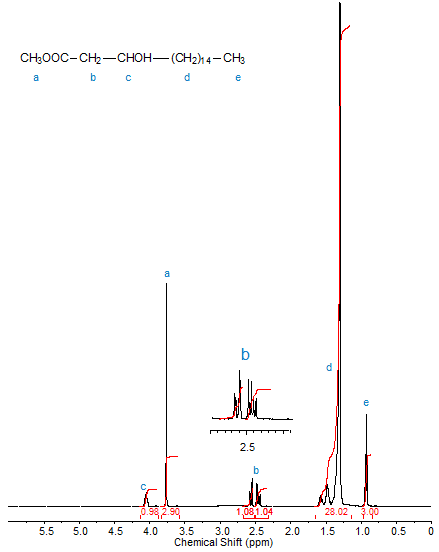
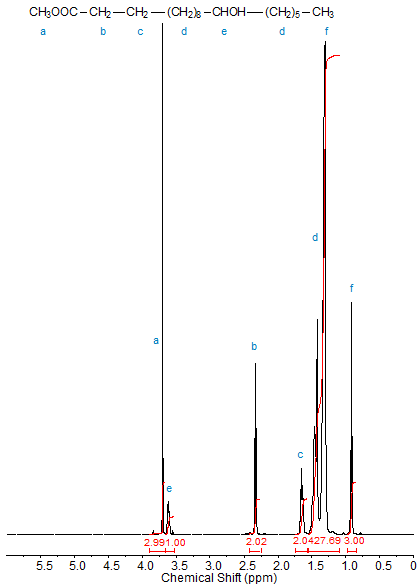
Figures 1, 2, and 3 depict the 1H-NMR spectra (in CDCl3) of methyl 2-hydroxyoctadecanoate, methyl 3-hydroxyoctadecanoate and methyl 12-hydroxyoctadecanoate, respectively. The progressive downfield shift of the proton attached to the hydroxyl-bearing carbon with increasing distance of the OH group from C1 is clearly visible. Note the splitting of the signals of diastereotopic protons such as those of C2 in methyl 3-hydroxyoctadecanoate.
Figure 1. 1H-NMR spectrum of methyl 2-hydroxyoctadecanoate.
Figure 2. 1H-NMR spectrum of methyl 3-hydroxyoctadecanoate.
Figure 3. 1H-NMR spectrum of methyl 12-hydroxyoctadecanoate.
Information on the NMR spectra of saturated hydroxy fatty compounds can be found in other publications. In (S)-(+)-methyl-3-hydroxyhexadecanoate, the proton of the hydroxy-bearing carbon at C-3 generated a multiplet at 3.98 ppm while the signal of the protons at the C-2 carbon was split into two doublets of doublets resonating at 2.39 and 2.50 ppm (Jakob et al., 1996).
Shift differences of the methine protons in vicinal hydroxy (1,2-diols) fatty acids allow distinguishing of the erythro and threo diastereomers (Ewing and Hopkins, 1967).
Literature:
- Ewing, D.F. and Hopkins, C.Y. Optical and geometric isomers of some fatty acids with vicinal hydroxy groups. Can. J. Chem., 45, 1259-1265 (1967).
- Jakob, B., Voss, G. and Gerlach, H. Synthesis of (S)- and (R)-3-hydroxyhexadecanoic acid. Tetrahedron: Asymmetry, 7, 3255-3262 (1996).
- Tulloch, A.P. Solvent effects on the nuclear magnetic resonance spectra of methyl hydroxystearates. J. Am. Oil Chem. Soc., 43, 670-674 (1966).
Unsaturated Hydroxy Fatty Acids
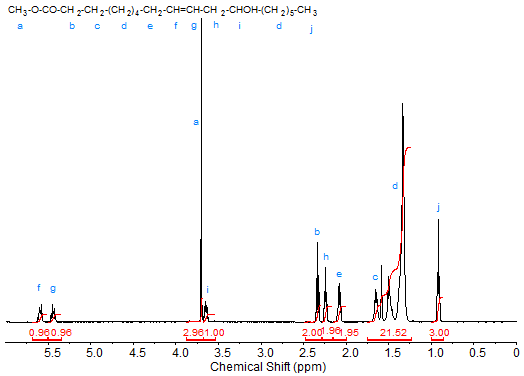
The prime example of an unsaturated hydroxy fatty acid is probably ricinoleic (12-hydroxy-octadeca-9-enoic) acid, the major fatty acid in castor oil, which contains a hydroxy group in the homoallylic position (3(Z)-enol). The C20 analogue of ricinoleic acid is lesqueroleic acid obtained from lesquerella oil. The proton NMR spectrum of the methyl ester of ricinoleic acid is shown in Figure 4. Due to the proximity of the hydroxy group to the double bond, the signal of the olefinic protons is split, with the downfield signal assigned to the proton at C9 and the upfield signal to the proton at C-10 (Lie Ken Jie and Cheng, 1993). The signal (quintet) of the proton at C12 is observed slightly upfield at about 3.65 ppm of the methyl ester signal, even slightly overlapping it. Again the protons at C2 are found at about 2.3 ppm. The presence of the hydroxy group causes the signals of the allylic protons to split compared to methyl oleate. The signals of the protons at C11 and C8 are observed at 2.20 and 2.05 ppm, respectively. The signals of the other protons are contained in the broad methylene signal, with the peaks of the protons at C3 and C13 being at the downfield end.
Figure 4. 1H-NMR spectrum of methyl ricinoleate.
7,10-Dihydroxy-8(E)-octadecenoic acid (DHOE) is an unusual product obtained by hydroxylation of oleic acid with certain Pseudomonas species (Parra et al., 1990, Hou et al., 1991). The erythro / threo diastereomers of corresponding synthetic allylic dihydroxy compounds (2(E)-ene-1,4-diols) can be distinguished by 1H-NMR in that the peak of the olefinic protons (5.60-5.68 ppm in deuteromethanol (CD3OD)) in the erythro diastereomers is slightly downfield (0.02-0.05 ppm) from that of the threo diastereomer (Knothe et al., 1994). In methyl 8,11-dihydroxy-9-octadecynoate, the shift of the protons attached to the hydroxy-bearing carbons was observed as triplet at 4.39 ppm.
In mono-unsaturated compounds (trans double bonds) with allylic hydroxy groups (2(E)-enols), the position (towards C1 or the terminal CH3) of the hydroxy group can be determined by the separation of the individual peaks within the multiplet caused by the olefinic carbons (Knothe et al., 1996). The shift differences are greater for position I (OH closer to C1) compounds, however, with increasing distance the shift differences in position II compounds decrease more rapidly. The differences can be described as rational functions. At about C13, the shift differences between position I and II disappear.
In the allenic compound methyl 13-hydroxy-(9Z,11E)-octadecadienoate, the protons of the double bonds resonated at 5.40 ppm (ddt, H-9), 5.64 ppm (dd, H-12), 5.94 ppm (dd, H-10), and 6.45 ppm (ddd, H-11) while the C-8 protons were observed as a multiplet at 2.14 ppm and the C-13 proton also caused a multiplet but at 4.13 ppm (Kuklev et al., 1997). Similar shifts were observed for 9-hydroxy-(10E,12Z)-octadecadienoate.
The 1H-NMR spectrum of methyl 12-hydroxy-9-octadecynoate showed a multiplet at 2.20 ppm caused by the propargylic protons at C-8 and C-11 and another multiplet at 3.96 ppm assignable to the C-12 proton (Lie Ken Jie et al., 1996). When adding another OH group to give methyl 11,12-dihydroxy-9-octadecynoate, a triplet at 2.20 ppm generated by the propargylic protons at C-8 was observed besides a multiplet at 3.59 ppm assigned to the protons at C-12, and a doublet at 4.12 ppm caused by the proton at C-11 (Lie Ken Jie and Alam, 2001).
In a monohydroxy compound with one double and one triple bond, methyl 8-hydroxy-11(E), 9a-octadecenynoate, the olefinic protons generated signals at as a doublet at 5.48 ppm (H-11) and a multiplet at 6.16 ppm (H-12), while the proton at C-8 was observed at 4.48 ppm, and the allylic protons at C-13 gave rise to a doublet of triplets at 2.08 ppm.
Literature:
- Hou, C.T. and Bagby, M.O. Production of a new compound, 7,10-dihydroxy-8(e)-octadecenoic acid, from oleic acid by Pseudomonas sp. PR3. J. Ind. Microbiol., 7, 123-129 (1991).
- Knothe, G., Bagby, M.O. and Weisleder, D. Evaluation of the olefinic proton signals in the 1H-NMR spectra of allylic hydroxy groups in long-chain compounds. Chem. Phys. Lipids, 82, 33-37 (1996).
- Knothe, G., Bagby, M.O., Weisleder, D. and Peterson, R.E. Allylic mono- and dihydroxylation of isolated double bonds with selenium dioxide/tert.-butylhydroperoxide. NMR characterization of long-chain enols, allylic and saturated 1,4-diols, and enones. J. Chem. Soc., Perkin Trans. 2, 1661-1669 (1994).
- Kuklev, D.V., Christie, W.W., Durand, T., Rossi, J.C., Vidal, J.P., Kasyanov, S.P., Akulin, V.N. and Bezuglov, V.V. Synthesis of keto- and hydroxydienoic compounds from linoleic acid. Chem. Phys. Lipids, 85, 125-134 (1997).
- Lie Ken Jie, M.S.F. and Cheng, A.K.L. Confirmation of the carbon chemical shifts of ethylenic carbon atoms in methyl ricinoleate and methyl ricinelaidate. Natural Prod. Lett., 3, 65-69 (1993).
- Lie Ken Jie, M.S.F., Pasha, M.K. and Ahmad, F. Ultrasound-assisted synthesis of santalbic acid and a study of triacylglycerol species in Santalum album (Linn.) seed oil. Lipids, 31, 1083-1089 (1996).
- Lie Ken Jie, M.S.F., Pasha, M.K. and Alam M.S. Oxidation reactions of acetylenic fatty esters with selenium dioxide/tert.-butylhydroperoxide. Lipids, 32, 1119-1123 (1997).
- Lie Ken Jie, M.S.F. and Alam, M.S. Novel azido fatty acid ester derivatives from conjugated C18 enynoate. Chem. Phys. Lipids, 111, 29-35 (2001).
- Parra, J.L., Pastor, J., Comelles, F., Manresa, M.A. and Bosch, M.P. Studies of biosurfactants obtained from olive oil. Tenside, Surfactants, Deterg., 27, 302-306 (1990).
Hydroperoxy Acids
Table 2 contains some data for two unsaturated hydroperoxy acids:
| Table 2. 1H-NMR (300 MHz, CDCl3) data of hydroperoxyoctadecenoic acids. | |||||||
| Double bond | Hydroperoxy position | —CH=CH— | —CH—OOH | ||||
|---|---|---|---|---|---|---|---|
| 10(E) | 9 | 5.76 (dt, H11), 5.35 (dd, H10) | 4.25 (dt, H9) | 2.07 (dt, H-8) | |||
| 8(E) | 10 | 5.76 (dt, H8), 5.35 (dd, H9) | 4.25 (dt, H-10) | 2.07 (dt, H-11) | |||
| Porter, N.A. and Wujek, J.S. Allylic hydroperoxide rearrangement: β-scission or concerted pathway? J. Org. Chem., 52, 5085-5089 (1987). | |||||||
Related Resources
Lipid Library
Edible Oil Processing
In the present context, the term edible oil processing covers the range of industrial…
Lipid Library
The Highs and Lows of Cannabis Testing
October 2016 With increasing legalization of both adult recreational and medical cannabis,…
Lipid Library
The secrets of Belgian chocolate
By Laura Cassiday May 2012 Like a bonbon nestled snugly in a…