Cyclic Fatty Acids
The Author: Frank D. Gunstone, James Hutton Institute (and Mylnefield Lipid Analysis), Invergowrie, Dundee (DD2 5DA), Scotland
Fatty acids containing a carbocyclic unit are most commonly cyclopropene or cyclopentene compounds (see our web page on natural cyclic fatty acids). Eicosanoids such as the prostaglandins are also cyclopentane derivatives but these are not included here, and acids with heterocyclic units are discussed elsewhere (see epoxy acids).
Cycopropenyl Fatty Acids
The best known cyclopropene acids are malvalic and sterculic which are present in high levels in sterculia oils and at lower levels in kapok seed oil (around 12%) and in cottonseed oil (about 1%). The highly reactive cyclopropene acids are destroyed during refining and hydrogenation of the oils. They have attracted interest because they inhibit the biological desaturation of stearic to oleic acid. 2-Hydroxysterculic acid, which has also been identified, is probably an intermediate in the bioconversion of sterculic to malvalic acid by α-oxidation.
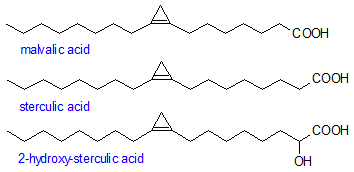
NMR data for the cyclopropene acids are presented in two Tables. Table 1 contains data for sterculic and malvalic acids and for natural and synthetic compound closely related to these. The compounds in the Table 2 are synthetic compounds with 14-16 or 24-26 carbon atoms. The cyclopropene unit itself has a methylene signal (7.0-7.5 ppm) and two olefinic signals. The latter differ by a value that is high when the cyclopropene unit is close to the carboxyl group and diminishes as it moves away. Figures in the two Tables show differences of 3.6 (Δ4), 2.5 (Δ5), 0.5 (Δ8), and 0.3 (Δ9). The cyclopropene unit also has a marked influence on the chemical shifts of nearby carbon atoms. Pollardhas given shielding parameters for the cyclopropene ring of -3.73 (α), -2.38 (β), -0.5 (γ), and -0.3 (Δ). These are larger than the corresponding parameters for the olefinic group.
| Table 1. Chemical shifts (ppm) for sterculic, malvalic, and related esters | |||||
| A | B | C | D | E | |
|---|---|---|---|---|---|
| CH2 | 7.247 | 7.46 | 7.43 | ||
| 1 | 173.233 | 172.819 | 173.204 | 175.93 | |
| 2 | 33.875 | 34.036 | 33.878 | 33.93 | 70.49 |
| 3 | 24.696 | 24.731 | 24.660 | 24.78 | 34.44 |
| 4 | 28.925 | 28.889 | 28.773 | 27.02 | 24.77 |
| 5 | 28.925 | 28.935 | 28.871 | 25.75 | |
| 6 | 29.069 | 29.080 | 27.196 | 108.88 | |
| 7 | 27.192 | 27.205 | 25.788 | 110.20 | 27.33 |
| 8 | 25.836 | 25.836 | 109.092 | 26.12 | 26.02 |
| 9 | 109.193 | 109.175 | 109.556 | 27.48 | 109.23 |
| 10 | 109.459 | 109.474 | 25.902 | 109.52 | |
| 11 | 25.897 | 25.897 | 27.255 | 26.10 | |
| 12 | 27.250 | 27.250 | 29.275 | 27.45 | |
| 13 | 29.274 | 29.274 | 29.243 | ||
| 14 | 29.265 | 29.265 | 29.243 | ||
| 15/ω-4 | 29.154 | 29.154 | 31.754 | 32.04 | 31.94 |
| 16/ω-3 | 31.747 | 31.747 | 22.561 | 22.77 | 22.74 |
| 17/ω-2 | 22.550 | 22.550 | 13.993 | 14.11 | 14.18 |
| 18/ω-1 | 13.979 | 13.979 | |||
A, sterculic glycerol ester (α-chain) [Howarth and Vlahov, 1996]; B, sterculic glycerol ester (β-chain where chemical shifts differ from those in the α-chain) [Howarth and Vlahov, 1996]; C, malvalic glycerol ester (α-chain) [Howarth and Vlahov, 1996]; D, 6,7 isomer of sterculic acid [Pollard, private communication]; E, 2-OH sterculic ester [Spitzer, 1991] corrected). |
|||||
| Table 2. Chemical shifts (ppm) for some synthetic cyclopropene esters | |||||||
| A | B | C | D | E | F | G | |
|---|---|---|---|---|---|---|---|
| CH2 | 7.43 | 7.39 | 7.54 | 6.92 | 6.98 | 6.99 | 6.92 |
| 1 | 173.88 | 174.03 | 173.51 | 174.33 | 174.37 | 174.23 | 174.33 |
| 2 | 33.56 | 33.88 | 32.20 | 34.11 | 34.12 | 34.12 | 34.10 |
| 3 | 22.82 | 24.70 | 21.98 | 24.95 | 24.93 | 24.96 | 24.94 |
| 4 | 25.40 | 26.94 | 107.61 | - | - | - | - |
| 5 | 108.17 | 25.68 | 111.18 | - | - | - | - |
| 6 | 110.65 | 108.72 | 25.94 | - | - | - | - |
| 7 | 26.02 | 110.00 | 27.35 | - | - | - | - |
| 8 | 27.38 | 26.03 | - | - | - | 27.08* | 27.38 |
| 9 | - | 27.42 | - | - | 26.01 | 26.01 | 25.99 |
| 10 | - | - | - | 27.37 | 25.69* | 109.22 | 109.07 |
| 11 | - | - | - | 26.01 | 109.18 | 109.33 | 109.32 |
| 12 | - | - | - | 108.99 | 109.18 | 26.01 | 28.06 |
| 13 | - | - | - | 109.34 | 25.98* | 27.36* | - |
| ω-3 | 31.97 | 31.98 | 31.98 | 28.06 | 27.34 | 31.62 | - |
| ω-2 | 22.71 | 22.73 | 22.75 | 20.73 | 22.42 | 22.45 | 20.72 |
| ω-1 | 14.16 | 14.12 | 14.18 | 13.99 | 13.83 | 14.01 | 13.97 |
|
In the following structures, cp represents the cyclopropene ring C3H2 |
|||||||
| A: CH3(CH2)17cp(CH2)3COOCH3 [Hartman] | E: CH3(CH2)3cp(CH2)9COOCH3 [Gosalbo] | ||||||
| B: CH3(CH2)17cp(CH2)4COOCH3 [Hartman] | F: CH3(CH2)4cp(CH2)8COOCH3 [Gosalbo] | ||||||
| C: CH3(CH2)17cp(CH2)2COOCH3 [Hartman] | G: CH3(CH2)2cp(CH2)8COOCH3 [Gosalbo] | ||||||
| D: CH3(CH2)2cp(CH2)10COOCH3 [Gosalbo] | |||||||
| *These two signals could be interchanged. | Compounds D-G are partly deuterated | ||||||
Cyclopentenyl Fatty Acids
Cyclopentene acids are present in the seed oils of the Flacourticeae. They range from C6 to C24 compounds with the structure shown. Some members have a second double bond in the side chain. The most common are hydnocarpic acid (16:1), chaulmoogric (18:1), and gorlic (18:2).
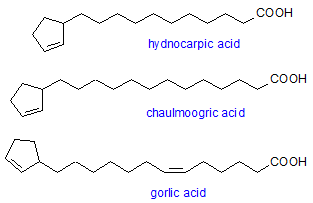
The NMR spectra of the cyclopentene acids lack the ω-1 to ω-3 signals characteristic of most fatty acids and instead show signals with unusual chemical shifts associated with the cyclopentene ring. Also present are signals at around 28, 29, and 36 ppm from the carbon atoms designated α, β, and γ in the above structure (Table 3).
| Table 3. Chemical shifts (ppm) for some cyclopentenyl esters | |||
| Chaulmoogric | Hydnocarpic | Gorlic | |
|---|---|---|---|
| 1 | 174.40 | 174.40 | 174.35 |
| 2 | 34.10 | 34.10 | 33.99 |
| 3 | 24.93 | 24.93 | 24.55 |
| 4 | 29.12 | 29.12 | 29.58 |
| 5 | 29.43 | 29.43 | 26.78 |
| 6 | 29.56 | 9.56 | 129.01 |
| 7 | 29.62 | 29.62 | 130.44 |
| 8 | 29.62 | 29.62 | 27.20 |
| 9 | 29.84 | - | 29.18 |
| 10 | 29.76 | - | 29.69 |
| γ | 27.97* | 27.97* | 27.94* |
| β | 29.23* | 29.23* | 29.23* |
| α | 36.15 | 36.15 | 36.13 |
| a | 45.58 | 45.58 | |
| b | 135.45 | 135.43 | 135.43 |
| c | 129.97 | 129.94 | 129.98 |
| d | 31.96 | 31.94 | 31.96 |
| e | 29.87 | 29.85 | 29.84 |
|
Chaulmoogric acid 18:1; hydnocarpic acid 16:1; gorlic acid 18:2. *These two signals could be interchanged |
|||
References
- Blaise, P., Fairnes, M. and Soulier, J. Identification of cyclopentenyl fatty acids by 1H and 13C nuclear magnetic resonance. J. Am. Oil Chem. Soc., 74, 727-730 (1997).
- Gosalbo, L., Barrot, M., Fabrias, G., Aesequell, G. and Camps, F. Synthesis of deuterated cyclopropene esters structurally related to palmitic and myristic acids. Lipids, 28, 1125-1130 (1993).
- Hartmann, S., Minnikin, D.E., Romming, H.-J., Baird, M.S., Ratledge, C. and Wheeler, P.R. Synthesis of methyl 3-(2-octadecylcyclopropen-1-yl)propanoate and methyl 3-(2-octadecylcyclopropen-1-yl)pentanoate and cyclopropane fatty acids as possible inhibitors of mycolic acid biosynthesis. Chem. Phys. Lipids, 71, 99-108 (1994).
- Howarth, O.W. and Vlahov, G. 13C Nuclear magnetic resonance study of cyclopropenoid triacylglycerols. Chem. Phys. Lipids, 81, 81-85 (1996).
- Spitzer, V. GC-MS characterisation (chemical ionisation and electron impact modes) of the methyl esters and oxazoline derivatives of cyclopropenoid fatty acids. J. Am. Oil Chem. Soc., 68, 963-69 (1991).
In This Section
- Introduction of NMR
- Saturated Fatty Acids and Methyl Esters
- Alkyl Esters Other than Methyl
- Glycerol Esters
- Non-Conjugated Double Bonds
- Conjugated Linoleic Acid (CLA)
- Acetylenic Fatty Acids and Derivatives
- Branched-Chain and Cyclic Fatty Acids
- Epoxy Fatty Acids
- Hydroxy and Hydroperoxy Fatty Acids
- Oxo Fatty Acids
- Fatty Alcohols
- Some Miscellaneous Fatty Acids
- Quantification by 1H-NMR
- The NMR Spectrum
- Alkanoic Acids
- Monoenoic Acids
- Polyunsaturated Fatty Acids
- Non-Methylene-Interrupted Polyenoic Fatty Acids
- Acids with conjugated unsaturation
- Acetylenic and Allenic Acids and Esters
- Branched-Chain and Cyclic Fatty Acids
- Cyclic Fatty Acids
- Epoxides and Acyclic Ethers
- Hydroxy and Hydroperoxy Acids
- Oxo (Keto) Acids
- Acids, Esters (Alkyl, Glycerol, Waxes), Alcohols and Acetates, Amides, and Nitriles
- Esters of Glycerol and Other Polyhydric Alcohols
- Oils and Fats
- Regiospecific Analysis of Triacylglycerols
