Acids, Esters (Alkyl, Glycerol, Waxes), Alcohols and Acetates, Amides, and Nitriles
The Author: Frank D. Gunstone, James Hutton Institute (and Mylnefield Lipid Analysis), Invergowrie, Dundee (DD2 5DA), Scotland
Chemical shifts (ppm) for several carbon atoms in oleic acid and some of its derivatives are summarised in the Table below. There are diagnostic signals for these classes of long-chain compounds.
| Carbon atom | a | b | c | d | e | f |
|---|---|---|---|---|---|---|
| Acid | 180.43 | 34.13 | 24.70 | - | - | - |
| Methyl ester | 174.28 | 34.10 | 25.01 | 51.39 | - | - |
| Glycerol ester (α) | 173.23 | 34.07 | 34.91 | - | - | - |
| Glycerol ester (β) | 172.82 | 34.23 | 24.93 | - | - | - |
| Alcohol | - | - | - | 63.03 | 32.81 | 25.76 |
| Acetate | 171.16 | 20.99 | - | 64.63 | 28.66 | 25.96 |
| Wax ester | 173.97 | 34.43 | 25.06 | 64.38 | 28.69 | 25.97 |
| Amide (i) | 76.37 | 36.02 | 25.57 | - | - | - |
| Nitrile (ii) | 119.77 | 25.41 | 17.12* | - | - | - |
| * Also 28.68 (C4) 28.77 (C5) (i) Huang et al. (1997) have given different values for the amide of 12-hydroxystearic acid: 176 (C1) and 33.1 (C2). (ii) Information on more complex nitriles is given in many papers devoted to the synthesis of polyunsaturated fatty acids since they are important intermediates. |
||||||
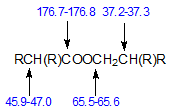
Characteristic chemical shifts have also been reported for di-Guerbet esters. These are branched-chain compounds made from Guerbet acids and Guerbet alcohols and have the structure shown below where the four R groups (C4 – C12) may differ from each other.
References
- Gunstone, F.D . High resolution 13C-NMR spectra of long-chain acids, methyl esters, glycerol esters, wax esters, nitriles, amides, alcohols, and acetates. Chem. Phys. Lipids, 66, 189-193 (1993).
- Huang, K.-K., Keudell, K.C., Klopfenstein, W.E., Wen, L., Bagby, M.O., Vesonder, R.F., Norton, R.A. and Weisleder, D. Biotransformation of 12-hydroxyoctadecanoic acid to 12-hydroxyoctadecanamide by Bacillus cereus 50. J. Am. Oil Chem. Soc., 74, 601-603 (1997).
- Knothe, G. and Carlson, K.D. Synthesis, mass spectrometry, and nuclear magnetic resonance characterization of di-Guerbet esters. J. Am. Oil Chem. Soc., 75, 1861-1866 (1998).
- Vieville, C., Mouloungui, Z. and Gaset, A. Synthesis and analysis of C1-C18 alkyl oleates. Chem. Phys. Lipids, 75, 101-108 (1995).
In This Section
- Introduction of NMR
- Saturated Fatty Acids and Methyl Esters
- Alkyl Esters Other than Methyl
- Glycerol Esters
- Non-Conjugated Double Bonds
- Conjugated Linoleic Acid (CLA)
- Acetylenic Fatty Acids and Derivatives
- Branched-Chain and Cyclic Fatty Acids
- Epoxy Fatty Acids
- Hydroxy and Hydroperoxy Fatty Acids
- Oxo Fatty Acids
- Fatty Alcohols
- Some Miscellaneous Fatty Acids
- Quantification by 1H-NMR
- The NMR Spectrum
- Alkanoic Acids
- Monoenoic Acids
- Polyunsaturated Fatty Acids
- Non-Methylene-Interrupted Polyenoic Fatty Acids
- Acids with conjugated unsaturation
- Acetylenic and Allenic Acids and Esters
- Branched-Chain and Cyclic Fatty Acids
- Cyclic Fatty Acids
- Epoxides and Acyclic Ethers
- Hydroxy and Hydroperoxy Acids
- Oxo (Keto) Acids
- Acids, Esters (Alkyl, Glycerol, Waxes), Alcohols and Acetates, Amides, and Nitriles
- Esters of Glycerol and Other Polyhydric Alcohols
- Oils and Fats
- Regiospecific Analysis of Triacylglycerols
