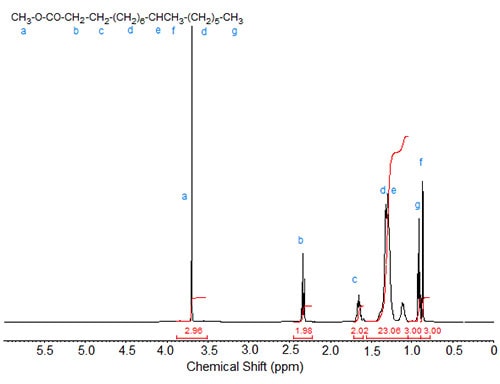
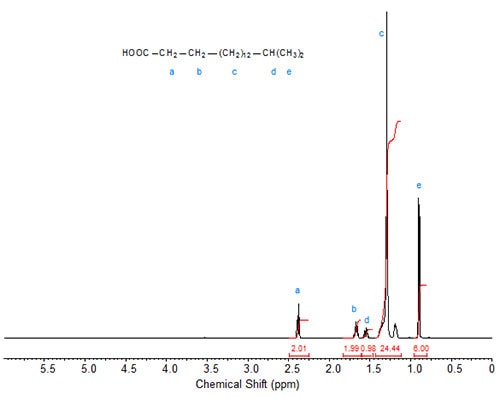
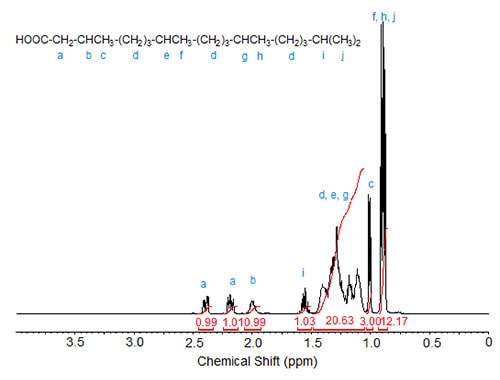
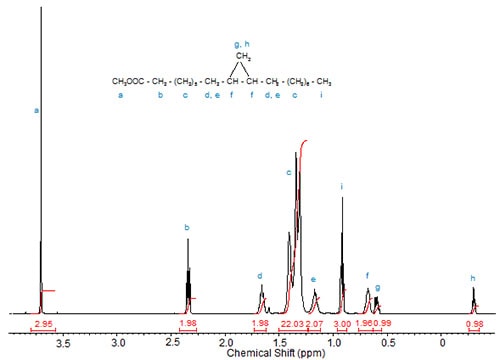
Figures 1 to 3 show the 1H-NMR spectra of several branched acids or esters. In methyl 10-methylhexadecanoate, the methyl group at C10 exhibits, as expected, a doublet, which is slightly upfield from the triplet of the terminal methyl group at C16 (Fig. 1). Accordingly, in isostearic acid (16-methyl heptadecanoic acid; Fig. 2), the two equivalent terminal methyl groups exhibit one doublet of integration value 6 at 0.9 ppm. The signal of the methine proton at C16 is observed at 1.55 ppm.
Figure 1. 1H-NMR spectrum of methyl 10-methylhexadecanoate.
Figure 2. 1H-NMR spectrum of 16-methyl-heptadecanoic (isostearic) acid.
In phytanic acid (3,7,11,15-tetramethylhexadecanoic acid; Fig. 3), the methyl group at C3 can be correlated with the doublet at 1.01 ppm while the other four methyl groups (the terminal methyl groups being equivalent) cause the two overlapping doublets of integration value 12 at 0.85-0.90 ppm. The complex C-H signal area with a theoretical integration value of 20 is caused by the nine methylene groups in the three CH2 clusters and the two methine protons at C7 and C11. The signal of the C15 methine proton is observed at 1.57 ppm (similar to the methine proton in isostearic acid) while the C3 methine proton causes the multiplet at 2.00 ppm. The diastereotopic protons at C2 show a split signal at 2.4 and 2.18 for one proton each.
Figure 3. 1H-NMR spectrum of phytanic acid.
An unsaturated acid with an allylic methyl group, 9-methyl-10(Z)-hexadecenoic acid, displayed the shifts of the methyl groups at 0.85 (triplet of terminal CH3) and 0.89 (doublet of the methyl branch), the signal of the proton at C-9 as multiplet at 2.31 ppm, those of the olefinic protons at 5.08 ppm (broad triplet, H-10) and 5.24-5.37 ppm (multiplet of H-11) as well as multiplet of H-12 at 1.99 ppm (Carballeira et al., 1999).
Similar data for branched fatty compounds can be obtained from the literature, for example, synthetic compounds (Biermann and Metzger, 2004) or unusual fatty acids found in the mollusc Siphonaria denticulata (Carballeira et al., 2001).
Literature
- Biermann, U. and Metzger, J.O. Alkylation of alkenes: sesquichloride-mediated hydro-alkyl additions with alkyl chloroformates and di-tert-butylpyrocarbonate. J. Am. Chem. Soc., 126, 10319-10330 (2004).
- Carballeira, N.M., Sostre, A. and Restituyo, J.A. Synthesis of racemic 9-methyl-10-hexadecenoic acid. Chem. Phys. Lipids, 97, 87-91 (1999).
- Carballeira, N.M., Cruz, H., Hill, C.A., De Voss, J.J. and Garson, M. Identification and total synthesis of novel fatty acids from the siphonarid limpet Siphonaria denticulata. J. Nat. Prod., 64, 1426-1429 (2001).
Cyclic Fatty Acids
In fatty acids containing a cyclopropene unit within the chain, the protons of the ring methylene were observed as a singlet at 0.73-0.75 ppm (Gosalbo et al., 1993, Hartmann et al., 1994). The signal at 1.57 ppm was caused by the protons of the methylene moieties β to the cyclopropene ring as well as those of C3 (Gosalbo et al., 1993) or these signals were contained in the strong methylene peak (Hartmann et al., 1994). The signal at 2.37 ppm was attributed to the four protons of the methylene moieties adjacent to the carbons carrying the cyclopropene ring (Gosalbo et al., 1993) or a peak at 2.37 ppm caused by the protons at C2 and those α to the cyclopropene ring.
Figure 4 depicts the 1H-NMR spectrum (in CDCl3) of methyl dihydrosterculate, which contains a cyclopropane ring. The signal of the cis methylene proton of the ring is shifted remotely downfield to below 0 ppm while that of the trans proton is located close to that of the methane protons in the ring. The signals of the protons attached to the carbons adjacent to the ring are also split at 1.175 and 1.40 ppm (Knothe, 2006).
Figure 4. 1H-NMR spectrum of methyl dihydrosterculate.
In fatty acids containing a terminal cyclopentene ring (chaulmoogric acid, gorlic acid, hydnocarpic acid), the signal of the olefinic protons of the cyclopentenyl moiety was identified at 5.68 ppm and that of the methine proton of the carbon attached to the cyclopentene ring and the fatty acid chain at 2.60 ppm (Blaise et al., 1997). The signal of the methylene protons in the fatty acid chain α to the cyclopentene ring was split, giving two signals at 1.25 and 1.35 ppm as was the signal of the methylene protons α to the carbon in the cyclopentene ring at 2.02 and 1.27 ppm. The two remaining methylene protons in the cyclopentene ring (α to the double bond) exhibited a peak at 2.30 ppm, overlapping that of C2 in the fatty acid chain. The spectra were obtained with deuterochloroform (CDCl3) at 400 MHz.
Literature:
- Blaise, P., Farines, M. and Soulier, J. Identification of cyclopentenyl fatty acids by 1H and 13C nuclear magnetic resonance. J. Am. Oil Chem. Soc., 74, 727-730 (1997).
- Gosalbo, L., Barrot, M., Fabrias, G., Aesequell, G. and Camps, F. Synthesis of deuterated cyclopropene esters structurally related to palmitic and myristic acids. Lipids, 28, 1125-1130 (1993).
- Hartmann, S., Minnikin, D.E., Römming, H.J., Baird, M.S., Ratledge, C. and Wheeler, P.R. Synthesis of methyl 3-(2-octadecylcyclopropen-1-yl)propanoate and methyl 3-(2-octadecylcyclopropen-1-yl)pentanoate and cyclopropane fatty acids as possible inhibitors of mycolic acid biosynthesis. Chem. Phys. Lipids, 71, 99-108 (1994).
- Knothe, G. NMR characterization of dihydrosterculic acid and its methyl ester. Lipids, 41, 393-396 (2006).
Related Resources
Lipid Library
Edible Oil Processing
In the present context, the term edible oil processing covers the range of industrial…
Lipid Library
The Highs and Lows of Cannabis Testing
October 2016 With increasing legalization of both adult recreational and medical cannabis,…
Lipid Library
The secrets of Belgian chocolate
By Laura Cassiday May 2012 Like a bonbon nestled snugly in a…