Triacylglycerol Biosynthesis in Eukaryotic Microalgae– the biological basis for the 3rd generation biofuel
The Author: Yonghua Li-Beisson, CEA/CNRS/Aix-Marseille University, CEA Cadarache, France
The Search for Alternative Energies
Due to the ever-increasing energy demand, diminishing fossil fuel reserves, global warming, and other environmental as well as economical concerns, alternative forms of energy that are renewable and sustainable are highly desired. Biological oils stored in biomass are energy rich, have structures that resemble fossil fuel, and are important precursors for making biodiesel. Soybean, rapeseed and palm oils are currently the major feedstocks for biodiesel production [1]. However converting existing agricultural land for biodiesel production is far from meeting the current demand and has also several associated controversial issues such as food versus fuel, environmental and social impact.
Microalgae as a platform for biofuel production
It is well known that oils can be produced in significant amounts by some microalgae [1,2]. Energy production based on microalgae is often called a third generation biofuel as opposed to biofuels from edible (first generation) and nonedible (second generation) plant parts. Economic and productivity analyses have revealed the high potential for producing fuels from microalgae compared to current biodiesel of plant origin [1]. Microalgae potentially provide several advantages including: (i) high biomass productivity (Table 1); (ii) microalgae are fed on carbon dioxide; this provides greenhouse gas mitigation benefits; (iii) ability to grow on nonagricultural land thus circumventing the issue of 'food versus fuel'; (iv) much higher efficiency in converting solar energy into chemical energy than plant counterparts [3]; and (v)mMicroalgae need much less water than plant crops. Depending on the species, microalgae can grow in freshwater, seawater or even wastewater.
| Table 1. Comparison of oil productivity of major crops and two microalgae [4] | |||
| Crop | Oil content per tonne of biomass (wt% dry mass) | Oil Production (t/ha/y) | Biomass yield (L/ha/y) |
|---|---|---|---|
| Oilseed rape (UK) | 40-44% (of seeds) | 1.4 | 1560 |
| Soya | 20% (of seeds) | 0.48 | 544 |
| Jatropha | 30% (of seeds) | 2.4 | 2,700 |
| Chlorella vulgaris | Up to 46% | 7.2 | 8,200 |
| Nannochloropsis | Up to 50% | 20-30 | 23,000-34,000 |
From microalgae oil to biofuel: a short history
The idea of making transportation fuel from microalgae has been around for over 50 years. Interests in seeking other liquid fuel versus the current fossil fuel have seen a roller-coaster style tightly tuned to economic/technical development, political interests and energy crises. The first interest in algal fuel triggered by the energy crisis in the 1970s was initiated in the U.S. under the program ‘Biodiesel from Algae’ [5]. This programme eventually stopped in the mid-1990s due to budget cuts, the cheap price of fossil fuel and the limitations in technological innovations. Recently, massive investments are coming from both public and private funds, notably a $600 million investment from the multi-international company, ExxonMobil. This renewed interest in algal biofuel is powered by the latest development and innovation in molecular genetics, genome sequencing, synthetic biology and lipidomic approaches.
Current barriers to a sustainable production of algal fuel
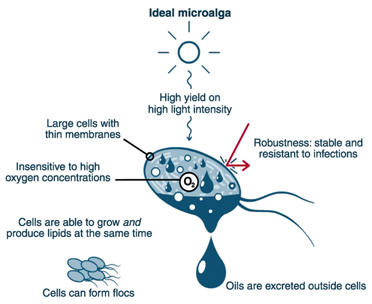
Despite all the advantages offered by microalgae, microalgal biofuel is not yet commercially feasible because it is still too expensive to produce. Algal fuel production involves mass cultivation, biomass harvest and drying, lipid extraction, and finally chemical transesterification for formation of biodiesel. We are still far from reaching the 'ideal algal fuel cell' (Fig. 1).
Figure 1. The ideal photosynthetic cell factory for production of biofuels (adapted from [6] with permission of the authors and publisher).
Concerning biology, one of the most obvious issues to solve is to increase lipid yields (the product of oil content and biomass productivity), which is currently fairly low. Indeed, most algae species start to accumulate considerable amount of lipids (up to 70% by dry weight) only when subjected to stresses, the most effective one being nitrogen removal [2]. However, nitrogen depletion limits biomass and therefore the overall lipid productivity. The lipid yields obtained so far are 10-20 times lower than the theoretical maximum [5]. For technology, the most notorious challenge lies in the field of biomass harvest and lipid extraction, which contribute to almost 70-80% of the total production cost [7].
Microalgae models: Chlamydomonas reinhardtii and beyond?
The green microalga Chlamydomonas reinhardtii is the best-developed unicellular green microalgae with sophisticated genetic tools [8]. C. reinhardtii is often called ‘the green yeast’ due to the power of its genetics. It has a short doubling time (8 hr), a fully sequenced genome [9], a large collections of EST datasets, and well-understood physiology. All three genomes of C. reinhardtii can be transformed routinely [10]. The easy transformation of the genome of its chloroplast (which is derived from cyanobacteria and where fatty acid synthesis takes place) will allow scientists to take advantage of the genetic studies conducted on plant chloroplasts and cyanobacteria. As a eukaryotic microalga, it has much higher lipid-synthesizing capacity than its prokaryotic counterpart, the cyanobacteria. C. reinhardtii has been used to study photosynthesis, starch synthesis, hydrogen production and more recently lipid metabolism (Fig. 2).
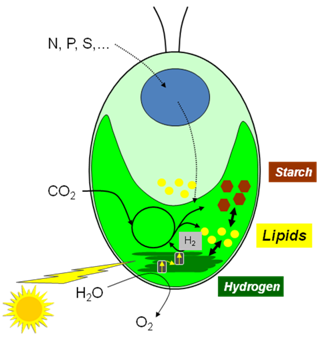
Figure 2. A schematic drawing of Chlamydomonas reinhardtii with its current use as a model for research on lipid, starch, bio-hydrogen production; also highlighted is the presence of one single cup-shaped chloroplast.
Chlamydomonas reinhardtii belongs to one group of green eukaryotic microalgae, called Chlorophyceae. Chlorophyceae is one of the many groups of oxygenic photosynthetic algae that represent an extremely diverse group of microorganism that are able to live in a large range of ecological habitats including freshwater, brackish, marine, saline, and a range of extreme pH and temperature conditions. This reflects their tremendous metabolic flexibility and the large range of organic compounds they produce. Compared to land plants, algae synthesize a much wider range of fatty acid and lipid molecular species (for detail see [11]). Algae produce not only the most common membrane lipids, but also storage lipids, wax esters, sterols, hydrocarbons, as well as quinones, tocopherols, etc. In this chapter, focus is given only to biosynthesis of one type of neutral lipid, the triacylglycerols.
C. reinhardtii has been selected as a laboratory strain for studying many fundamental biological processes and metabolisms, and it has not been 'cultivated' for any industrial purposes. Thus, besides C. reinhardtii, several other microalgal species are emerging as models for algal lipid production. These microalgal species include several species of Nannochloropsis, Chlorella, Ostreococcus and also a diatom Phaeodactylum tricornutum. Nannochloropsis have gained strong interest due to the fact that these species are exemplary biomass and lipid producers, and the possibility of generating null mutants based on homologous recombination [12], which is not possible with C. reinhardtii.
Lipids of Chlamydomonas reinhardtii
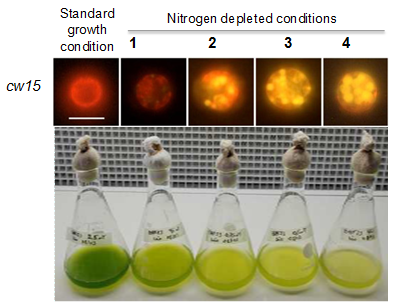
C. reinhardii produces triacylglycerols in the form of oil bodies in response to stress, such as nutrient deprivation, high light, and high salinity. So far the most effective trigger for oil accumulation is nitrogen removal. The accumulation of oil droplets in nitrogen-starved cells of C. reinhardtii can be visualized by staining with a neutral lipid specific dye Nile red. By using this dye, the kinetics of lipid accumulation upon nitrogen stress can be followed (Fig. 3). The fatty acid and lipid compositions were characterized in detail in the 1980s by the classical work of Giroud and co-workers [13-15] (Fig. 4).
Figure 3. Oil accumulation process in the model microalga Chlamydomonas reinhardtii, as followed by Nile red staining (upper panel) and visual observation (panel down). cw15: cell wall-less strain 15. Data are partly based on [17].
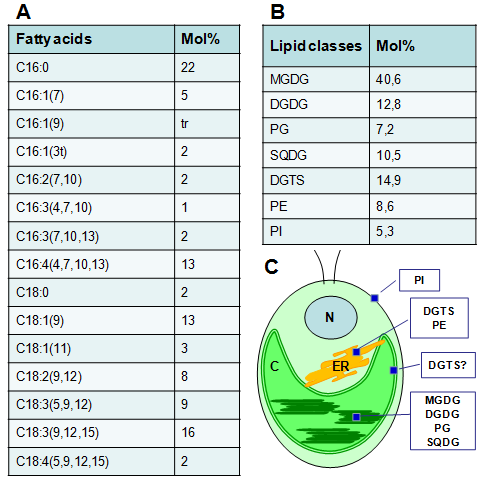
Figure 4: Fatty acid (A) and lipid composition (B) of Chlamydomonas reinhardtii and a sketch outline of the general features of C. reinhardtii with the location of the major lipid classes indicated (C). Fatty acids are denoted as the number of carbon atoms, followed by the number (and position) of double bond. Data source is based on that of Giroud et al., 1988 [14].
Abbreviations: N: Nucleus; ER, Endoplasmic reticulum; C, Chloroplast; MGDG, monogalactosyldiacylglycerol; DGDG, digalactosyldiacylglycerol; SQDG, sulfoquinovosyldiacylglycerol; PG, phosphatidylglycerol; PE, phosphatidylethanolamine; PI, phosphatidylinositol; DGTS, diacylglyceroltrimethylhomoserine.
A few features mark the difference in lipid composition between Chlamydomonas and land plants:
-
Besides the common membrane lipids present in most land plants, notably monogalactosyldiacylglycerol, digalactosyldiacylglycerol, phosphatidylglycerol, and phosphatidylethanolamine, Chlamydomonas membrane lipids are characterized by the absence of phosphatidylcholine (PC) – the major cytoplasmic lipids in land plants - but the presence of a large quantity of diacylglyceroltrimethylhomoserine (DGTS), a betaine lipid. DGTS is has structural similarities to PC, thus is generally thought to replace cellular PC for biological functions.
-
Fatty acids in Chlamydomonas usually have similar acyl chain lengths (C16-C18) to those present in most land plant species. However, contrary to oleaginous plants where 1 or 2 double bonds are common, fatty acids with 3 and 4 double bonds are abundant, which make the oil more fluid, but also more susceptible to oxidation.
-
The presence of unconventional non-methylene-interrupted pinolenic acid C18:3(5,9,12) and coniferonic acid C18:4 (5,9,12,15), which are rarely present in plant species. The gene encoding the novel desaturase has been cloned and shown to be able to produce these fatty acids in transgenic yeasts [16].
The Pathway of Oil Biosynthesis: from CO2 to Triacylglycerols
Extensive knowledge on oil synthetic pathways is gained mostly from studies of plant or animal models. Several related topics are covered in this series, including fatty acid biosynthesis, triacylglycerol biosynthesis and triacylglycerol mobilization. Our understanding of oil synthesis in algae is still fragmentary. Nevertheless, based on genome comparison and protein homology search, it is generally believed that the basic pathways of fatty acid and lipid synthesis are conserved in the green lineage. In a photosynthetic cell, oil synthesis requires hundreds of enzymatic reactions and occurs in several subcellular compartments. To put it in a linear way, oil biosynthesis involve three independently regulated and physically separated steps, i.e. de novo fatty acid synthesis in the plastid, glycerolipid assembly in the endoplasmic reticulum (ER) and its final packaging into the oil bodies (as outlined in Fig. 5).
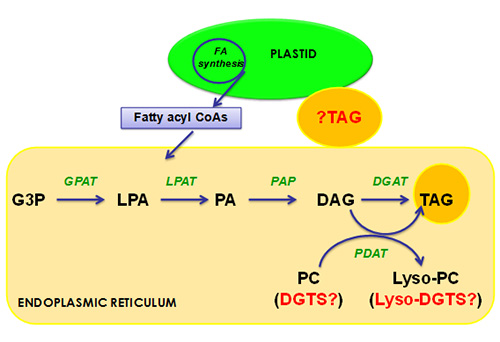
Figure 5: The three parallel pathways leading to formation of triacylglycerols in a single cell of Chlamydomonas reinhardtii. In red are unknowns, which require further experimental verification. A plastidial pathway has recently been reported for Chlamydomonas and the exact enzymes involved are not yet characterized.
Abbreviations: DAG, diacylglycerol; ER, endoplasmic reticulum; GPAT, glycerol-3-phosphate acyltransferase; G3P, glycerol-3-phosphate; LPAT, lysophosphatidic acid acyltransferase; LPA, lysophosphatidic acid; PA, phosphatidic acid; PAP, phosphatidic acid phosphatase; TAG, triacylglycerol; DGTS, diacylglyceroltrimethylhomoserine.
De novo fatty acid synthesis in the plastids
Microalgae can fix CO2 into sugars using energy from the sun. The fixed sugars are further processed to produce acetyl-CoA (coenzyme A), and more than one pathway may contribute to maintain the acetyl-CoA pool. Acetyl-CoA provided by photosynthesis serves as the precursor for fatty acid synthesis in the chloroplast. Fatty acids are the building blocks of many cellular lipid types including triacylglycerols. The first committed step of fatty acid synthesis is catalyzed by a multifunctional enzyme complex, i.e. acetyl CoA carboxylase (ACCase), which produces malonyl-CoA from acetyl CoA and bicarbonate. ACCase has been cloned and characterized from two eukaryotic microalgal species. Before being further used by the fatty acid synthase machinery, the malonyl group is transferred from CoA to ACP (acetyl carrier protein) catalyzed by a malonyl-CoA:acyl carrier protein malonyltransferase. The common 16- or 18-carbon fatty acids are formed by a series of two-carbon chain-elongating reactions catalyzed by a multisubunit enzyme named fatty acid synthase (FAS). Fatty acid synthesis requires stoichiometric amount of ATP, acetyl CoA and NADPH for each two carbons added to the growing acyl chain. Photosynthetic reactions are thus essential not only in providing a carbon source but also in generating reducing power (NADH and NADPH) and energy (ATP) for fatty acid synthesis.
For most algae species, the final acyl chains emerging from the chloroplast are 16 or 18 carbons in length. The chain-elongating reaction is terminated by the action of acyl-ACP thioesterase (FAT). The specificity of this enzyme usually determines the final chain length of the product. The released free fatty acids can cross the plastid envelope membrane where they are esterified to CoA via another enzymatic reaction catalyzed by long-chain acyl-CoA synthases (LACS).
Acyl-lipid assembly in the endoplasmic reticulum (ER) and in the plastids
It is generally accepted that triacylglycerols are formed by the sequential acylation of sn-glycerol-3-phosphate (G3P) backbone with three acyl-CoAs catalyzed by a group of enzymes named acyltransferases. This pathway first described by Professor Eugene Kennedy and colleagues in the 1950s is also known as the 'Kennedy Pathway'. This pathway and its related route from acyl-CoA to oil synthesis have recently been reviewed in 'acyl-lipid metabolism' as an Arabidopsis Book chapter [20]. Biochemical activities and enzymes of this pathway have often been found associated to the membranes of the endoplasmic reticulum where it is accepted as the cellular location for glycerolipid assembly. It is initiated by the acylation of the sn-1 position of G3P by an sn-glycerol-3-phosphate acyltransferase (GPAT) to produce lysophosphatidic acid (LPA). LPA is then further acylated by the action of lysophosphatidic acid acyltransferase (LPAT) to form phosphatidic acid (PA). Phosphatidic acid phosphatase (PAP) catalyzes the removal of the phosphate group from phosphatidic acid to generate sn-1,2-diacylglycerol (DAG), the central intermediate of all glycerolipids. The last and committed step to oil synthesis is catalyzed by diacylglycerol acyltransferase (DGAT). The above mentioned three acyltransferases all use acyl-CoA as acyl donor and these reactions are thus acyl-CoA-dependent.
An alternative route to oil synthesis that is present in both plants and yeast is catalyzed by phospholipid:diacylglycerol acyltransferase (PDAT) contributing to the synthesis of triacylglycerol using phosphatidylcholine as an acyl donor and sn-1,2-diacylglycerol as an acyl acceptor. The ortholog of the gene encoding this enzyme is present in some sequenced algal genomes including Chlamydomonas reinhardtii, therefore suggesting the likely presence of this pathway in green algal lineage.
Fan and Xu [21] have recently demonstrated that at least part of the triacylglycerol synthetic pathway is present in the plastid of C. reinhardtii, and they have further observed under transmission electron microscopy (TEM) that some of the oil droplets formed in the plastid can be secreted and relocated to the cytosol. This is confirmed by another report by the laboratory of Professor Ursula Goodenough [19]. These lines of evidence point out the fact that in Chlamydomonas reinhardtii at least some triacylglycerols are made in the plastid (Fig. 5). These findings are significant and require further confirmation via biochemical approaches.
Triacylglycerol packing into oil bodies
After a certain amount of triacylglycerols form in specific domain of ER, droplets of oil bud off of the ER, forming distinct cellular organelles. Due to their highly hydrophobic nature, oils could not exist alone in the cell. Their dispersion in the cytoplasm is facilitated by the fact that they are enclosed by a single-layer membrane made of phospholipids with the hydrophilic head groups on the surface. These subcellular compartments are called oil bodies, lipid droplets or oleosomes. Oil bodies are spherical organelles consisting of a neutral lipid core enclosed by a membrane lipid monolayer coated with proteins. Huang has presented a concise overview on oil bodies of plant origin. Modern mass spectrometry has revealed the presence of many proteins in the isolated oil body fraction. Until now major structural proteins of oil bodies found include oleosins in oilseeds, perilipin in adipocytes, and major lipid droplet protein (MLDP) present in three microalgal chlorophytes Chlamydomonas reinhardtii, Haematococcus pluvialis and Dunaliella.
Besides structural proteins, oil droplet-associated proteins include also lipid metabolic enzymes including acyltransferases, acyl-lipid exchange enzymes and lipase [18,22]. Numerous lipid-trafficking proteins are also present indicating the dynamic nature of Chlamydomonas oil bodies. These findings are consistent with the observation that oil bodies can be used rapidly when growth conditions become better, such as re-availability of a nitrogen source. This serves as another line of evidence that microalgae species have gained remarkable metabolic flexibility during evolution.
From Cellular Oil Bodies to Biodiesel
So the question now is how to convert the cellular oil to drive a car? First of all, we will need to get the oil (= triacylglycerols) into a fairly pure form; this will involve oil extraction, which includes separation of the oil from other biomass materials such as cell wall, protein and starch. Industrial oil extraction and purification technologies already exist for plant oils, which therefore can be adapted for algal oil (see the Processing web pages). Analytical techniques are covered in the section of this web site on Lipid Analysis.
As discussed above, algal oils are primarily composed of various triacylglycerols consisting of fatty acid chains (usually 16 or 18 carbons long) esterified to glycerol. The fatty acyl chains are chemically similar to the hydrocarbons that make up the bulk of the molecules found in diesel (usually 10-15 carbons long). However, most algal/plant oil has a viscosity range that is much higher than that of conventional diesel, thus algal oil cannot be used directly as biofuel; another process called transesterification is required. Transesterification of triacylglycerols can be acid- or base-catalyzed in the presence of methanol to produce corresponding fatty acid methyl esters. It is this mixture of monoalkyl fatty acid esters collectively that is called biodiesel.
As well as fatty acyl chain length, the number of double bonds also plays a critical role in the final utility of such an oil. Contrary to diesel, naturally occurring fatty acids often harbor one to three double bonds. If too much saturated fatty acid is present, biodiesel turns into a gel, even at ambient temperatures; on the other hand, if too much unsaturated fatty acid is present, the biodiesel produced will have good cold flow properties but is prone to oxidation. Engineering efforts toward producing oils with shorter chain length and fewer double bonds would be useful.
Room for Genetic Engineering?
- from functional genomics to commercial reality
Paul Roesller and co-workers [23-25] have tested the effect of native overexpression of acyl-CoA desaturase (ACCase) in Cyclotella cryptica on cellular oil content. Although this study did not increase oil content in the host algal strain, it serves as the first example of a genetic engineering study of lipid genes in a eukaryotic microalga. Recently, with expanding development in technology, several proteomic [18,22] and comparative transcriptomic studies [26] have been carried out in algal strains under normal and oil-accumulating conditions. These studies have provided a rich source of genes, enzymes, potential regulatory proteins for further detailed functional characterization. For biotechnological purposes, two major targets are described here:
Increasing oil yield
Approaches for increasing oil yield have included overexpression of native genes, identification of transcriptional control factors or introduction of novel genes from exotic species. Current efforts include forward genetic screening approaches, as well as '-omics' approaches in identifying key regulators or transcriptional factosr of oil synthetic pathways in microalgae.
The observation of massive formation of oil droplets upon nitrogen stress led to speculation on the existence of a nitrogen-related genetic 'trigger'. Genetic attempts to identify this trigger include forward genetic screening programs and comparative transcriptomic approaches. Comparative transcriptomic studies of normal and nitrogen-limited conditions have been carried out for several algal species including C. reinhardtii, P. tricornutum, and Thalassiosira pseudonana [26,27]; this has resulted in identification of a putative transcription factor that acts as a lipid trigger in C. reinhardtii, and the authors have further demonstrated that overexpression of this gene led to an approximately 50% increase in lipid production [28].
Altering fatty acid composition
Oil properties are determined by its acyl composition: both carbon chain length and the number of double bonds (i.e. degree of unsaturation). Naturally occurring microalgal species synthesize a vast range fatty acid structures [11]. Biodiesel with shorter chain-length fatty acids (C10-C12) have improved cold flow properties; thus, isolating genes encoding shorter chain-specific acyl-ACP thioesterases could be useful for reducing the chain length of fatty acyl groups in the oil of microalgae and are of great biotechnological interest. The first successful example of such an engineering effort has been proved in a diatom where research scientists at Colorado School of Mines (USA) overexpressed two genes encoding acyl-ACP thioesterase of plant origin in P. tricornutum to produce medium-chain fatty acids in the oil fraction [29].
Closing Comments
The widespread interest and massive investment in microalgal fuel have stimulated the investigation of lipid biosynthesis in these organisms. Key factors involved in oil accumulation in microalgae are being identified. With the new tools developed in the field of imaging, lipidomics, systems biology, and genetic engineering, we can expect new breakthroughs in algal lipid research in the near future.
References
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv., 25, 294-306 (2007) (DOI: 10.1016/j.biotechadv.2007.02.001).
- Hu, Q., Sommerfeld, M., Jarvis, E., Ghirardi, M., Posewitz, M., Seibert, M. and Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J., 54, 621-639 (2008) (DOI: 10.1111/j.1365-313X.2008.03492.x).
- Beer, L.L., Boyd, E.S., Peters, J.W. and Posewitz, M.C. Engineering algae for biohydrogen and biofuel production. Curr. Opin. Biotechnol., 20, 264-271 (2009) (DOI: 10.1016/j.copbio.2009.06.002).
- Scott, S.A., Davey, M.P., Dennis, J.S., Horst, I., Howe, C.J., Lea-Smith, D.J. and Smith, A.G. Biodiesel from algae: challenges and prospects. Curr. Opin. Biotechnol., 21, 277-286 (2010) (DOI: 10.1016/j.copbio.2010.03.005).
- Sheehan, J., Dunahay, T., Benemann, J. and Roessler, P.G. A look back at the US Department of Energy’s aquatic species program – biodiesel from algae. (Ed. C.O .Golden, National Renewable Energy Laboratory, US Department of Energy’s Office of Fuels Development) (1998) (www.nrel.gov/docs/legosti/fy98/24190.pdf).
- Wijffels, R.H. and Barbosa, M.J. An outlook on microalgal biofuels. Science, 329, 796-799 (2010) (DOI: 10.1126/science.1189003).
- Grima, E.M., Belarbi, E.H., Fernandez, F.G.A., Medina, A.R. and Chisti, Y. Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol. Adv., 20, 491-515 (2003) (DOI: 10.1016/S0734-9750(02)00050-2).
- Harris, E.H. The Chlamydomonas sourcebook: introduction to Chlamydomonas and its laboratory use. 2nd Edition. (Elsevier) (2009).
- Merchant, S.S., Prochnik, S.E., Vallon, O., Harris, E.H., Karpowicz, S.J., Witman, G.B., Terry, A., Salamov, A., Fritz-Laylin, L.K., Marechal-Drouard, L., et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science, 318, 245-250 (2007) (DOI: 10.1126/science.1143609).
- Rochaix, J.-D. Chlamydomonas, a model system for studying the assembly and dynamics of photosynthetic complexes. FEBS Lett., 529, 34-38 (2002) (DOI: 10.1016/S0014-5793(02)03181-2).
- Harwood, J.L. and Guschina, I.A.The versatility of algae and their lipid metabolism. Biochimie, 91, 679-684 (2009) (DOI: 10.1016/j.biochi.2008.11.004).
- Kilian, O., Benemann, C.S.E., Niyogi, K.K. and Vick, B.High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp. Proc. Natl. Acad. Sci. U.S.A., 108, 21265-21269 (2011) (DOI: 10.1073/pnas.1105861108).
- Giroud, C. and Eichenberger, W. Lipids of Chlamydomonas-reinhardtii - incorporation of 14C-acetate, 14C-palmitate and 14C-oleate into different lipids and evidence for lipid-linked desaturation of fatty-acids. Plant Cell Physiol., 30, 121-128 (1989).
- Giroud, C. and Eichenberger, W. Fatty-acids of Chlamydomonas reinhardtii - structure, positional distribution and biosynthesis. Biol. Chem. Hoppe-Seyler, 369, 18-19 (1988).
- Giroud, C., Gerber, A. and Eichenberger, W. Lipids of Chlamydomonas-reinhardtii - analysis of molecular-species and intracellular site(s) of biosynthesis. Plant Cell Physiol., 29, 587-595 (1988) (pcp.oxfordjournals.org/content/29/4/587).
- Kajikawa, M., Yamato, K.T., Kohzu, Y., Shoji, S., Matsui, K., Tanaka, Y., Sakai, Y. and Fukuzawa, H. A front-end desaturase from Chlamydomonas reinhardtii produces pinolenic and coniferonic acids by omega-13 desaturation in methylotrophic yeast and tobacco. Plant Cell Physiol., 47, 64-73 (2006) (DOI: 10.1093/pcp/pci224).
- Siaut, M., Cuine, S., Cagnon, C., Fessler, B., Nguyen, M., Carrier, P., Beyly, A., Beisson, F., Triantaphylides, C., Li-Beisson, Y. et al. Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol., 11, 7 (2011) (DOI: 10.1186/1472-6750-11-7).
- Nguyen, H.M., Baudet, M., Cuiné, S., Adriano, J.-M., Barthe, D., Billon, E., Bruley, C., Beisson, F., Peltier, G., Ferro, M. et al. Proteomic profiling of oil bodies isolated from the unicellular green microalga Chlamydomonas reinhardtii: With focus on proteins involved in lipid metabolism. Proteomics, 11, 4266-4273 (2011) (DOI: 10.1002/pmic.201100114).
- Goodson, C., Roth, R., Wang, Z.T. and Goodenough, U. Structural correlates of cytoplasmic and chloroplast lipid body synthesis in Chlamydomonas reinhardtii and stimulation of lipid body production with acetate boost. Eukaryot. Cell, 10, 1592-1606 (2011) (DOI: 10.1128/EC.05242-11).
- Li-Beisson, Y.S.B., Beisson, F., Andersson, M., Arondel, V., Bates, P., Baud, S., Bird, D., DeBono, A., Durrett, T., Franke, R., Graham, I., Katayama, K., Kelly, A., Larson, T., Markham, J., Miquel, M., Molina, I., Nishida, I., Rowland, O., Samuels, L., Schmid, K., Wada, H., Welti, R., Xu, C., Zallot, R. and Ohlrogge, J. Acyl lipid metabolism. In: The Arabidopsis Book. (Ed. R. Last, American Society of Plant Biologists) (2010) (DOI: 10.1199/tab.0133).
- Fan, J.L., Andre, C. and Xu, C.C. A chloroplast pathway for the de novo biosynthesis of triacylglycerol in Chlamydomonas reinhardtii. FEBS Lett., 585, 1985-1991 (2011) (DOI: 10.1016/j.febslet.2011.05.018).
- Moellering, E.R. and Benning, C. RNA interference silencing of a major lipid droplet protein affects lipid droplet size in Chlamydomonas reinhardtii. Eukaryot. Cell, 9, 97-106 (2010) (DOI: 10.1128/EC.00203-09).
- Roessler, P.G., Bleibaum, J.L., Thompson, G.A. and Ohlrogge J.B. Characteristics of the gene that encodes acetyl-CoA carboxylase in the diatom Cyclotella cryptica. Ann. N.Y. Acad. Sci., 721, 250-256 (1994) (10.1111/j.1749-6632.1994.tb47398.x).
- Roessler, P.G. and Ohlrogge, J.B. Cloning and characterization of the gene that encodes acetyl-coenzyme-A carboxylase in the alga Cyclotella cryptica. J. Biol. Chem., 268, 19254-19259 (1993) (www.jbc.org/content/268/26/19254).
- Dunahay, T.G, Jarvis, E.E. and Roessler, P.G. Genetic transformation of the diatoms Cyclotella cryptica and Navicula saprophila. J. Phycol., 31, 1004-1012 (1995) (DOI: 10.1111/j.0022-3646.1995.01004.x).
- Miller, R., Wu, G.X., Deshpande, R.R., Vieler, A., Gartner, K., Li, X.B., Moellering, E.R., Zauner, S., Cornish, A.J., Liu, B.S. et al. Changes in transcript abundance in Chlamydomonas reinhardtii following nitrogen deprivation predict diversion of metabolism. Plant Physiol., 154, 1737-1752 (2010) (DOI: 10.1104/pp.110.165159).
- Maheswari, U., Montsant, A., Goll, J., Krishnasamy, S., Rajyashri, K.R., Patell, V.M. and Bowler, C. The diatom EST database. Nucleic Acids Res., 33, D344-D347 (2005) (DOI: 10.1093/nar/gki121).
- Yohn, C.M.M., Behnke, C. and Brand, A. Stress-induced lipid trigger. US Patent (2011).
- Radakovits, R., Eduafo, P.M. and Posewitz, M.C. Genetic engineering of fatty acid chain length in Phaeodactylum tricornutum. Metab. Engin., 13, 89-95 (2011) (DOI: 10.1016/j.ymben.2010.10.003).
In This Section
- Plant Fatty Acid Synthesis
- Production of Unusual Fatty Acids in Plants
- Arabidopsis Acyl-Coenzyme A-Binding Proteins
- Long Chain acyl-coA Synthetases and Other Acyl Activating Enzymes
- Plant Triacylglycerol Synthesis
- Triacylglycerol Biosynthesis in Eukaryotic Microalgae
- Subcellular Oil Droplets and Oleosins in Plants
- Triacylglycerol Mobilisation in Plants
- Role of Transcription Factors in Storage Lipid Accumulation in Plants
- Biosynthesis of Plant Lipid Polyesters
- Rubber Biosynthesis in Plants
- Carotenoid Biosynthesis and Regulation in Plants
- The Oxylipin Biosynthetic Pathways in Plants
- N-Acylphosphatidylethanolamines (NAPEs), N-acylethanolamines (NAEs) and Other Acylamides: Metabolism, Occurrence and Functions in Plants
- Phosphoinositide Signaling in Plants
- Plant Lipidomics
- 50 years of Galactolipid Research: The Beginnings
- Transport and function of lipids in the plant phloem