Phosphoinositide Signaling in Plants
The Author: Ingo Heilmann, Department of Cellular Biochemistry, Institute for Biochemistry and Biotechnology, Martin-Luther-University Halle-Wittenberg, Kurt-Mothes-Str. 3, 06120 Halle (Saale), Germany
Introduction
Phospholipids are important building blocks of biological membranes in all eukaryotes. The main body of most membrane bilayers is composed of structural membrane lipids, such as phosphatidylcholine or phosphatidylethanolamine. Besides such structural components, there are also lipids of only low abundance or transient occurrence, but with nonetheless important regulatory roles, such as phosphoinositides (PIs). Instead of having structural roles, PIs are involved in various cellular processes, including the control of membrane trafficking, cytoskeletal remodeling, ion transport and signal transduction. This article is aimed at summarizing the roles of PIs in plant function and development that have been uncovered so far.
Phosphoinositides Are Derived from Phosphatidylinositol
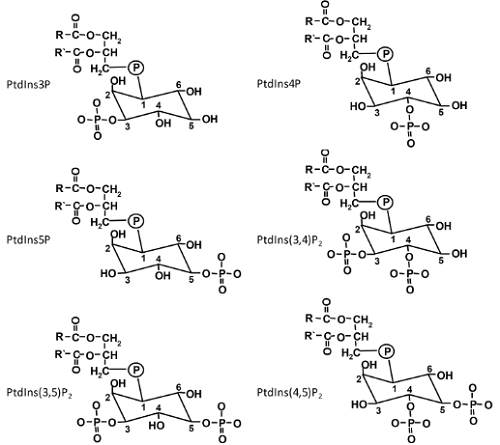
Phosphatidylinositol (PtdIns), the precursor of all PIs, is generated by the condensation of cytidine-diphosphodiacylglycerol and D-myo-inositol catalyzed by PtdIns synthases [1]. A. thaliana contains two isoforms, which are both localized in the endoplasmic reticulum (ER) with their catalytic centers likely oriented to the cytosolic face of the ER membrane. PIs derive from PtdIns by phosphorylation of the inositol headgroup. The inositol ring of PtdIns can be phosphorylated at the D-3, D-4 or D-5 positions by specific lipid kinases that, so far, have only been found in eukaryotes. The various phosphorylations can be carried out in consecutive steps, giving rise to a total of six structurally related PIs (Fig. 1). For instance, phosphatidylinositol-4-phosphate (PtdIns4P) can be formed from PtdIns by PI 4-kinases, or phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) from PtdIns4P by PI4P 5-kinases. A seventh PI, PtdIns(3,4,5)P3, has so far only been reported in animal cells. PIs exhibit pronounced asymmetry between membrane leaflets with the vast majority present in the cytosolic leaflet.
Figure 1. The six phosphoinositides found in plants.
Phosphoinositides Can Act as Ligands to Partner Proteins
Part of the regulatory effects PIs have in eukaryotic cells - including plants - is mediated by PIs acting as specific ligands to partner proteins. For instance, PIs can bind to integral membrane proteins and regulate their activity, as has been demonstrated for some plant ion channels and ATPases [1]. Binding of PIs can also recruit cytosolic proteins to a membrane, thus preventing or enabling their biochemical activity, as has been shown for enzymes involved in cytoskeletal control. Binding of proteins to the negatively charged inositolpolyphosphate head groups can be electrostatic and involve protein domains rich in basic side chains.
More specific binding to partner proteins can also occur via the characteristic inositolpolyphosphate head groups of PIs, which have evolved to be recognized by specific PI-binding domains. Examples for such PI-binding domains are Pleckstrin homology (PH)-domains, the Fab1p-YOPB-Vps27p-EEA1 (FYVE)-domain or the Phox homology (PX)-domain, which can each bind to different PIs [2]. A large number of proteins encoded in eukaryotic genomes contain specific PI-binding domains, suggesting that a wide variety of cellular processes involves PI-binding as part of relevant control mechanisms [2]. An interesting complication is presented by the observation that, e.g., PH-domains capable of binding PtdIns(4,5)P2 might only form conditionally when two proteins interact that each carry part of a functional PH-domain [3]. Because such partial PI-binding domains present in the individual protein sequences are not easily recognized by sequence analysis, this concept suggests that the number of PI-binding proteins - and also of physiological processes controlled by PIs - might be even larger than previously projected. In the future, progress in the study of the plant interactome and refined computer-aided sequence analysis might aid the identification of so-far-unrecognized PI-binding protein complexes in plants.
Phosphoinositides Might Exert Effects on the Biophysical Properties of Membranes
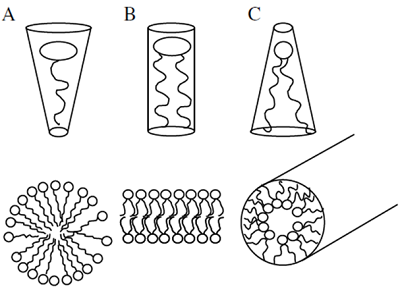
Besides interactions with partner proteins, the localized accumulation of PIs can exert effects on the biophysical properties of membranes. Membranes can be organized in near-planar areas or in ruffled areas characterized by increased local membrane curvature. It has been demonstrated that membrane lipids with a high degree of unsaturation in their fatty acid moieties will accumulate in areas of high curvature. The presence of such lipids also favors the formation of membrane vesicles. A helpful model for the explanation of the influence of lipid structure on membrane properties is the geometric approximation of lipid shape (Fig. 2).
Figure 2. Geometrical models of lipid shapes. A, inverse conical lipid, e.g., lysolipids, phosphoinositides (PIs); B, cylindrical lipid, e.g. phosphatidylcholine; C, conical lipid, e.g. phosphatidic acid. Bottom panels, simplified views of membrane curvature facilitated by lipids with different geometric shapes.
According to this model, lipids with roughly equal diameters of head group and fatty acid moiety, such as phosphatidylcholine, will be considered cylindrical, whereas lipids with small headgroups, such as phosphatidic acid, are conical and lipids with large headgroups, such as PIs, are inverse-conical [4]. In a planar membrane mostly built from cylindrical lipids the introduction of lipids with conical or inverse-conical geometries might induce curvature strain. Considering that PIs are exclusively associated with the cytosolic leaflets of membrane bilayers, it follows that PIs might dictate areas of increased membrane curvature, which would facilitate vesicle budding towards the cytosol during endocytosis or stabilize transient stages of vesicle fusion of secretory vesicles with the plasma membrane during exocytosis. Studies on the physiological roles of PIs in eukaryotic cells - including plants - support the notion that PIs are involved in vesicle budding and fusion during membrane trafficking, as will also be described further on. Because PIs can mediate the recruitment of vesicle coat proteins that will additionally affect and stabilize membrane curvature, such as clathrin, it remains unresolved whether PIs influence membrane curvature predominantly through biophysical effects or via protein recruitment, or both.
Phosphoinositides Can Serve as Precursors for Soluble Second Messengers
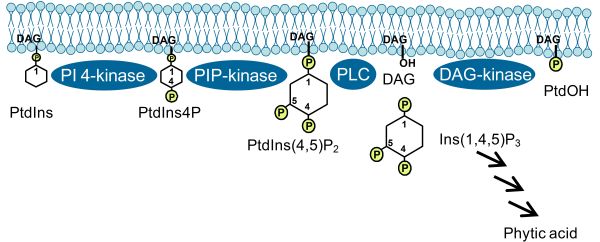
Roles of PIs described in previous paragraphs pertained to those exerted by the intact lipids. In addition, cleavage of PIs by phospholipase C (PLC) can also occur, for instance in response to environmental stresses (Fig. 3). While obviously the removal of PIs from the membrane in this fashion might restrict intact PIs in time and space and, thus, exert certain effects, it has been demonstrated that the cleavage products, diacylglycerol (DAG) and soluble inositol polyphosphates (IPPs), can experience further conversion, yielding potent second messengers in their own right [5]. For instance, DAG can be further phosphorylated by DAG-kinase to form phosphatidic acid, a lipid with many reported regulatory functions in plants [5]. Furthermore, IPPs can be phosphorylated to phytic acid (InsP6), which has been demonstrated to have a variety of important roles in eukaryotic cells, including the control of nuclear transcription or the nucleo-cytoplasmic export of mRNA. Importantly, recent evidence indicates that phytic acid occurs as a cofactor in the auxin-receptor protein, transport inhibitor response 1 (TIR1), an observation of so far unknown relevance for plant auxin perception [6]. Future studies will be aimed at elucidating the cross-talk of PI-derived signaling molecules with phytohormone cascades in plants [7].
Figure 3. PI pathway leading to the formation of PtdIns(4,5)P2 and formation of derived second messengers. PLC, phospholipase C; DAG, diacylglycerol; phytic acid, Ins(1,2,3,4,5,6)P6; PtdOH, phosphatidic acid. Arrows indicate subsequent steps of phosphorylation catalyzed by specific inositol polyphosphate kinases.
Roles of Phosphoinositides in Plant Stress Responses
Stress-induced changes in PI production have been reported for plants in numerous studies, suggesting roles for PIs in plant stress adaptation. For instance, different PIs change in abundance and localization in response to hyperosmotic stress in plants [5]. It has been reported that PtdIns3P acts in adaptive processes to salt stress by mediating endocytosis and membrane internalization. Recent biochemical and cell biological evidence suggests additional roles for salt stress-induced PtdIns(4,5)P2 in the formation of clathrin-coated vesicles and possibly stress-induced plasma membrane internalization. So far, the targets of stress-induced PIs and the particular physiological processes affected are not well understood, as functional evidence for the involvement of PIs in stress-induced membrane trafficking is still missing.
Roles for PIs in plant stress signaling are further supported by the characterization of plants in which PI metabolism has been perturbed by genetic manipulation. For instance, increased accumulation of PtdIns(4,5)P2 in A. thaliana plants deficient in the SAC-domain PI-phosphatase, SAC9, has been shown to correlate with constitutively induced stress responses. In contrast, attenuated responsiveness to stress has been reported for A. thaliana plants ectopically overexpressing a PI phosphatase [8]. Moreover, the targeted suppression of IPP signals by heterologous expression of the human type I inositol polyphosphate 5-phosphatase in A. thaliana resulted in decreased levels of Ins(1,4,5)P3 and attenuated responses to stimulation [9,10] and increased resistance to drought [11]. Interestingly, plants overexpressing PI phosphatases were not obviously altered in growth or development under non-challenging conditions, suggesting that no essential cellular function of PIs was affected by increased hydrolysis of IPPs.
Roles of Phosphoinositides in the Control of Polar Tip Growth in Plants
Root hairs and pollen tubes are established model systems for the study of polar tip growth in eukaryotic cells. In root hairs and pollen tubes, PIs control directional membrane trafficking required for the delivery of cell wall material and membrane area to the growing tip. For instance, A. thaliana mutants deficient in the production of PtdIns4P by PI 4-kinases β1 and β2 exhibited reduced root hair formation [12]. Similar observations were made for mutants deficient in the production of PtdIns(4,5)P2 by PIP 5K3 [1]. Exocytotic vesicles destined to fuse with the apical plasma membrane associate with PI 4-kinase β [12] and are likely coated with PtdIns4P, the preferred substrate for the PI4P 5-kinase, PIP5K3. Together, these data suggest that PtdIns4P and PtdIns(4,5)P2 act in a common pathway, possibly defining the cellular sites of exocytotic vesicle fusion required for polar tip growth in root hairs.
Pollen tubes are structurally similar to root hairs, and tip growth in both cell types is regulated by similar mechanisms. The hypothesis that increased levels of PtdIns4P and/or PtdIns(4,5)P2 result in a loss of cellular polarity of tip-growing cells is supported by data from pollen tubes, where dominant-negative effects with overexpression of inactive PLC proteins caused morphological alterations that could originate from increased levels of PtdIns(4,5)P2 [13,14]. PtdIns(4,5)P2 has been implicated in the control of vesicle trafficking in pollen tubes of A. thaliana and of Nicotiana tabacum [15,16] and in particular in the apical secretion of pectin [15]. PI 4-kinases of the β subfamily also occur in pollen tubes, and it was demonstrated that PI 4-kinase β1 and the PI4P 5-kinase, PIP5K5, act synergistically in a common pathway to mediate apical pectin secretion in pollen tubes [17].
Different Functions of Phosphoinositides Might Be Mediated by Distinct Phosphoinositide Pools
Emerging evidence indicates that the spatiotemporal distribution of PIs enables interactions with some partner proteins, while excluding others [18,19]. As a consequence, the subcellular localization of PIs might determine which of several alternative functions will be performed, manifesting a particular effect on physiology. However, neither the factors controlling the localization of PI-biosynthetic enzymes nor those defining the further distribution of PIs are well understood.
A given PI species, for instance PtdIns(4,5)P2, can be represented by different molecular species that differ in their associated fatty acids [20], affecting the lateral mobility of the lipids within the membrane and possibly directing them towards alternative interaction partners [21,22]. Although the fatty acid moieties of PIs have previously been largely ignored, future studies using new analytic techniques could reveal the nature of PI-molecular species associated with particular physiological processes. An example is the report that PtdIns(4,5)P2 species present constitutively in A. thaliana leaves and those formed upon hyperosmotic stress treatment differ in their associated fatty acids, as revealed by gas chromatographic analysis of fatty acids released from PIs re-isolated after thin-layer chromatography [20]. It is possible that different molecular species of PIs originate from different species of PtdIns formed by isoforms of PI synthase [1]. The variation in lateral mobility of different molecular species of PIs adds another dimension to the already-complex network of PI interrelations.
Fluorescent Reporters for Phosphoinositides: Cell Biology vs. Biochemical Analysis
The introduction of fluorescent reporters specifically decorating particular PIs enables the spatiotemporal dynamics of PI microdomains to be visualized in living cells. Although originally developed for use in mammalian model systems [23], reporters specific for PtdIns3P, PtdIns4P, or PtdIns(4,5)P2 have successfully been applied in plants to visualize lipid distribution [1]. The fluorescent reporters now available are based on the specific PI binding by protein domains originating from several proteins, as was already mentioned above. For example, by expressing a GFP-tagged PH domain of the human PLCδ1 in tobacco pollen tubes, it was shown that PtdIns(4,5)P2 forms a microdomain in the apical plasma membrane of pollen tube cells. A similar PtdIns(4,5)P2 microdomain has also been visualized in the plasma membrane of root hair tips. Other protein modules used to visualize PI-distribution are the FAPP-domain and the FYVE-domain with specificities for PtdIns4P and PtdIns3P, respectively. The combined expression of lipid-binding domains specific for different PIs has enabled the dynamic distributions of PtdIns3P, PtdIns4P and PtdIns(4,5)P2 in dividing plant cells to be imaged simultaneously, suggesting nonredundant roles for all three PIs in the formation of the cell plate [24].
A serious drawback of using lipid-binding domains as specific reporters is that specific binding of the expressed reporters to PIs can prevent the lipids from performing their regulatory functions in the cell. Although leading to compensatory increases in PI biosynthesis and, thus, at first being considered an artifact limiting interpretation [25], some studies have utilized the specific masking of PIs by lipid-binding domains for functional studies. For instance, by using this approach it was possible to demonstrate a role for PtdIns3P in vesicle trafficking in root hairs. Reciprocally, binding of PIs to their natural interaction partners might prevent the fluorescent reporter from decorating PIs. Based on a comparison of in vivo imaging and electron microscopy and immunodetection after fixation, it has been estimated that up to 60% of PtdIns(4,5)P2 present in a cell may not be decorated in vivo by the fluorescence-tagged PH domain of the human PLCδ1 [26]. Nonetheless, within the limits of interpretation, the use of fluorescent reporters specific for certain PIs provides a powerful tool with which to analyze PI signaling in plants.
Open Questions in the Plant Phosphoinositide Field
Previous work was mainly directed at identifying enzymes of PI biosynthesis and studying the effects of over- and underexpression in plants. The described physiological effects must now be rationalized by understanding the nature of downstream effects of PIs. To this end, it will be necessary to identify PI-binding proteins by proteomics approaches and define the detailed modes of action in subsequent studies. Another major question is that for the endogenous mechanisms regulating PI biosynthesis, which in the cell is often transient and spatially limited. Factors recruiting enzymes of PI biosynthesis into the correct signaling contexts of a target membrane are also not understood and must be studied. Here, it is likely that protein-protein interactions as well as protein-lipid interactions are of importance.
Overall, genetic, analytical and imaging methods from different fields of plant science will have to be combined to elucidate the workings of the plant PI network in all its complexity. In recent years the PI system has emerged to be of central importance for plant function. It is now an important goal to further the understanding of the PI system to enable new possibilities to modulate the growth of plants in the field, including plants of economic value.
References
- Heilmann, I. Using genetic tools to understand plant phosphoinositide signalling. Trends Plant Sci., 14, 171-179 (2009).
- Lemmon, M.A. Phosphoinositide recognition domains. Traffic, 4, 201-213 (2003).
- van Rossum, D.B., Patterson, R.L., Sharma, S., Barrow, R.K., Kornberg, M., Gill, D.L. and Snyder, S.H. Phospholipase Cγ1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature, 434, 99-104 (2005).
- Kooijman, E.E., Chupin, V., de Kruijff, B. and Burger, K.N. Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic, 4, 162-174 (2003).
- Meijer, H.J. and Munnik, T. Phospholipid-based signaling in plants. Annu. Rev. Plant Biol., 54, 265-306 (2003).
- Tan, X., Calderon-Villalobos, L.I., Sharon, M., Zheng, C., Robinson, C.V., Estelle, M. and Zheng, N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature, 446, 640-645 (2007).
- Mosblech, A., Feussner, I. and Heilmann, I. Oxylipins: structurally diverse metabolites from fatty acid oxidation. Plant Physiol. Biochem., 47, 511-517 (2009).
- Sanchez, J.P. and Chua, N.H. Arabidopsis PLC1 is required for secondary responses to abscisic acid signals. Plant Cell, 13, 1143-1154 (2001).
- Perera, I.Y., Love, J., Heilmann, I., Thompson, W.F. and Boss, W.F. Up-regulation of phosphoinositide metabolism in tobacco cells constitutively expressing the human type I inositol polyphosphate 5-phosphatase. Plant Physiol., 129, 1795-1806 (2002).
- Perera, I.Y., Hung, C.Y., Brady, S., Muday, G.K. and Boss, W.F. A universal role for inositol 1,4,5-trisphosphate-mediated signaling in plant gravitropism. Plant Physiol., 140, 746-760 (2006).
- Perera, I.Y., Hung, C.Y., Moore, C.D., Stevenson-Paulik, J. and Boss, W.F. Transgenic Arabidopsis plants expressing the type 1 inositol 5-phosphatase exhibit increased drought tolerance and altered abscisic acid signaling. Plant Cell, 20, 2876-2893 (2008).
- Preuss, M.L., Schmitz, A.J., Thole, J.M., Bonner, H.K., Otegui, M.S. and Nielsen, E. A role for the RabA4b effector protein PI-4Kβ1 in polarized expansion of root hair cells in Arabidopsis thaliana. J. Cell Biol., 172, 991-998 (2006).
- Dowd, P.E., Coursol, S., Skirpan, A.L., Kao, T.H. and Gilroy, S. Petunia phospholipase c1 is involved in pollen tube growth. Plant Cell, 18, 1438-1453 (2006).
- Helling, D., Possart, A., Cottier, S., Klahre, U. and Kost, B. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell, 18, 3519-34 (2006).
- Ischebeck, T., Stenzel, I. and Heilmann, I. Type B phosphatidylinositol-4-phosphate 5-kinases mediate pollen tube growth in Nicotiana tabacum and Arabidopsis by regulating apical pectin secretion. Plant Cell, 20, 3312-3330 (2008).
- Sousa, E., Kost, B. and Malho, R. Arabidopsis phosphatidylinositol-4-monophosphate 5-kinase 4 regulates pollen tube growth and polarity by modulating membrane recycling. Plant Cell, 20, 3050-3064 (2008).
- Ischebeck, T., Vu, L.H., Jin, X., Stenzel, I., Löfke, C. and Heilmann, I. Functional cooperativity of enzymes of phosphoinositide conversion according to synergistic effects on pectin secretion in tobacco pollen tubes. Mol. Plant, 1-12 (2010).
- Braun, M., Baluska, F., von Witsch, M. and Menzel, D. Redistribution of actin, profilin and phosphatidylinositol-4, 5-bisphosphate in growing and maturing root hairs. Planta, 209, 435-443 (1999).
- Kost, B., Lemichez, E., Spielhofer, P., Hong, Y., Tolias, K., Carpenter, C. and Chua, N.H. Rac homologues and compartmentalized phosphatidylinositol 4, 5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J. Cell Biol., 145, 317-330 (1999).
- König, S., Mosblech, A. and Heilmann, I. Stress-inducible and constitutive phosphoinositide pools have distinct fatty acid patterns in Arabidopsis thaliana. FASEB J., 21, 1958-1967 (2007).
- Cho, H., Kim, Y.A. and Ho, W.K. Phosphate number and acyl chain length determine the subcellular location and lateral mobility of phosphoinositides. Mol. Cells, 22, 97-103 (2006).
- Mukherjee, S., Soe, T.T. and Maxfield, F.R. Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails. J. Cell Biol., 144, 1271-1284 (1999).
- Varnai, P. and Balla, T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol., 143, 501-510 (1998).
- van Leeuwen, W., Vermeer, J.E., Gadella, T.W., Jr. and Munnik, T. Visualization of phosphatidylinositol 4,5-bisphosphate in the plasma membrane of suspension-cultured tobacco BY-2 cells and whole Arabidopsis seedlings. Plant J., 52, 1014-1026 (2007).
- Balla, T., Bondeva, T. and Varnai, P. How accurately can we image inositol lipids in living cells? Trends Pharmacol. Sci., 21, 238-241 (2000).
- Watt, S.A., Kular, G., Fleming, I.N., Downes, C.P. and Lucocq, J.M. Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C δ1. Biochem. J., 363, 657-666 (2002).
In This Section
- Plant Fatty Acid Synthesis
- Production of Unusual Fatty Acids in Plants
- Arabidopsis Acyl-Coenzyme A-Binding Proteins
- Long Chain acyl-coA Synthetases and Other Acyl Activating Enzymes
- Plant Triacylglycerol Synthesis
- Triacylglycerol Biosynthesis in Eukaryotic Microalgae
- Subcellular Oil Droplets and Oleosins in Plants
- Triacylglycerol Mobilisation in Plants
- Role of Transcription Factors in Storage Lipid Accumulation in Plants
- Biosynthesis of Plant Lipid Polyesters
- Rubber Biosynthesis in Plants
- Carotenoid Biosynthesis and Regulation in Plants
- The Oxylipin Biosynthetic Pathways in Plants
- N-Acylphosphatidylethanolamines (NAPEs), N-acylethanolamines (NAEs) and Other Acylamides: Metabolism, Occurrence and Functions in Plants
- Phosphoinositide Signaling in Plants
- Plant Lipidomics
- 50 years of Galactolipid Research: The Beginnings
- Transport and function of lipids in the plant phloem