Long Chain acyl-coA Synthetases and Other Acyl Activating Enzymes
The Author: Jay Shockey, Commodity Utilization Research Unit, Southern Regional Research Center, United States Department of Agriculture, Agricultural Research Service, New Orleans, LA, USA 70124
Introduction
Proper synthesis and breakdown of molecules containing carboxylic acids is a vital part of metabolism in all living organisms. Given the relatively inert chemical nature of many carboxylic acids, activation is a necessary step prior to use in the various anabolic and catabolic pathways that utilize these acids. Lipids, amino acids, sugars, cutin, suberin, glucosinolates, and various other secondary metabolites are built in part using activated carboxylic acids. There is immense variation in the size and structure of organic acids; it is not surprising that most organisms have evolved large families of enzymes that activate them. Collectively, these enzymes use a variety of compounds to activate the carboxylate group; however, the largest of the enzyme families is the acid-thiol ligases (EC 6.2.1). The most common thiol compound used in these reactions is coenzyme A, and the enzymes are generally categorized as CoA ligases or CoA synthetases.
The CoA synthetase reaction proceeds through a two-step mechanism involving the conversion of the carboxylate and ATP to an enzyme-bound carboxyl-AMP intermediate (called an adenylate) with the release of pyrophosphate (PPi). Then, the activated carbonyl carbon of the adenylate is coupled to the thiol of CoA, followed by enzyme release of the thioester and AMP. Amines or alcohol groups can also act as the nucleophile in this reaction (Schmelz and Naismith, 2009).
While both steps of the reaction are reversible, and have been demonstrated in vitro, PPi hydrolysis by pyrophosphatase in vivo drives the equilibrium to the right, and makes the reaction irreversible:
Figure 1. The generalized reaction scheme for acyl-CoA synthetases.
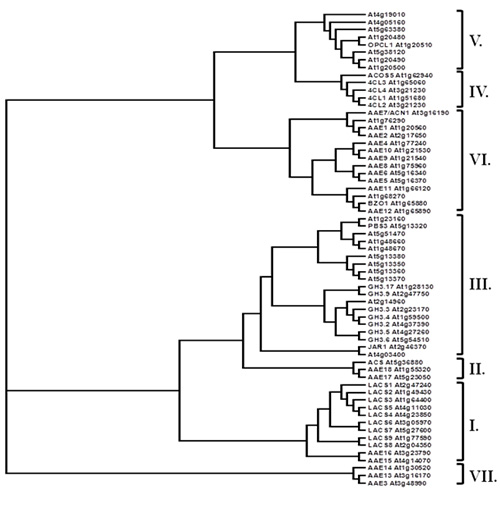
Most CoA synthetases and related proteins share a relatively modest level of amino acid identity across the length of the proteins, but are easily identified by a 12 amino acid residue domain called the AMP-binding motif (PROSITE PS00455). This motif was the key criterion used to designate these genes as Acyl Activating Enzymes (AAE) and also served as an important tool in the identification of the large AAE gene superfamily present in Arabidopsis (Shockey et al., 2003), Chlamydomonas reinhardtii, Populus trichocharpa, and Physcomitrella patens (Shockey and Browse, 2011). The AAE superfamily is made up of seven clades (Fig. 2), some of which are not represented in certain species of plants (Shockey and Browse, 2011). Several current reports have helped to determine substrate specificities, subcellular targeting and other properties of particular AAE enzymes.
Figure 2. The AAE gene superfamily in Arabidopsis thaliana. Clades (as designated in Shockey et al., 2003) are bracketed and marked at right. Locus identifiers and common names, where appropriate, are included.
Currently, more than a dozen microbial, animal, and plant proteins (Gulick, 2009; Hu et al., 2010) have been crystallized and their three-dimensional structures resolved. Modeling of proteins complexed to various ligands, including coenzyme A and mimics of the acyl-adenylate intermediate, have shed light on the catalytic mechanisms that are required for the two-step reaction (reviewed in Gulick, 2009).
In addition to the mechanistic insights, the crystallographic studies highlighted several residues that are necessary for proper substrate binding and catalysis. Many of the residues corresponding to binding of ATP and CoA are well conserved (described in detail in Gulick, 2009). However, the carboxylate binding site is located in the highly variable N-terminal domain, and accurate prediction of acyl substrate specificity based on primary amino acid sequence of this domain is extremely difficult. The substrates of this superfamily are very diverse in chain length and overall structure, and the core region of the N-terminal domain shares only about 5% amino acid identity, even within a small subset of structurally related bacterial acyl-CoA synthetases (Shah et al., 2009). Ongoing efforts to devise knowledge-based methods to deal with this uncertainty (Hu et al., 2010; Khurana et al., 2010) have allowed for prediction of likely substrates for certain groups of enzymes, but unambiguous determination of substrate preference for individual enzymes in the absence of functional testing will likely remain a daunting challenge for some time to come.
The past several years have seen great progress in the elucidation of the biochemical and physiological roles of representative enzymes from each of the seven clades of the AAE superfamily. Many recent discoveries have also refined our understanding of the general roles of previously characterized AAE proteins. Equally important though, enzymes have been identified that filled in long-standing gaps in our knowledge of well-characterized biochemical pathways, provided access to known pathways that were previously intractable to traditional genetic or biochemical approaches, and in some cases, even introduced the scientific community to reactions and pathways that were not previously known. A brief summary of some of these findings will be presented in the following sections.
The Biochemical Activities of AAE Enzymes Suggest Participation in a Wide Range of Physiological Processes
Known roles of “core” LACSs and other AAEs
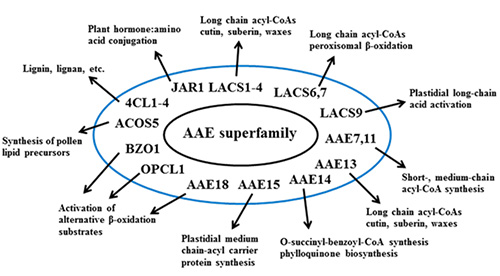
The seminal work of Fulda and colleagues (Fulda et al., 1994) led to the discovery of the first plant AAE genes, including long-chain acyl-CoA synthetases and other members of the group, and established the framework used to search the rapidly expanding Arabidopsis genome database for other related genes. This led to the classification of the family of nine genes encoding the long-chain acyl-CoA synthetases in Arabidopsis (Shockey et al., 2002) and later, to the identification of the entire 63 member AAE superfamily (Shockey et al., 2003). Many known roles for acyl-CoA synthetase activity had been established in the past, and the identification of these sets of genes provided the means by which to begin to connect certain genes with particular functions. Figure 3 provides a graphical representation of many of the known roles of AAE proteins. This work has been extensively reviewed recently (de Azevedo-Sauza et al., 2008; Shockey and Browse, 2011) for those seeking more details; a brief recap of these findings is presented here.
Figure 3. Cartoon diagram of the various known and suggested roles for various plant AAE proteins. See text below for more details and references to primary reports.
Fatty acid biosynthesis
Plastids are the site of de novo fatty acid synthesis in plant cells. The growing fatty acyl chains are maintained and elongated as thioesters to acyl carrier protein (ACP). The 16- and 18-carbon fatty acid final products are cleaved by thioesterase activities in the plastid and exported from the plastids as acyl-CoAs, for use in the cytosol in the various pathways that utilize these substrates. Previous studies unambiguously established that acyl-CoA thioesterase activity is found in the inner envelope of the chloroplast membrane, while acyl-CoA synthetase activity was located in the outer envelope. Identification of the LACS gene family in Arabidopsis provided a way to investigate which of the LACS isoform(s) might encode this activity. The expression patterns of LACS9 suggested that this gene might be a good candidate, and in vitro import assays established that the LACS9 protein was indeed targeted to chloroplasts (Schnurr et al., 2002). Enzyme assays using protein extracts from wild-type and lacs9 mutant plastids showed that LACS9 encodes approximately 90% of total ACS activity in this organelle. The identity of the enzyme that encodes the remaining 10%, which, surprisingly, is enough to maintain normal growth and development of lacs9 mutant plants (Schnurr et al., 2002), remains an enigma. LACS8 is the closest paralog to LACS9. However, multiple studies show that unlike LACS9, LACS8 is targeted to the ER (Dunkley et al., 2006; Zhao et al., 2010), and the combination of loss-of-function mutations in both LACS8 and LACS9 showed no additional phenotypic changes relative to the lacs9 mutant alone (Zhao et al., 2010). More work remains to be done to clarify which proteins constitute the complete plastidial LACS complement and what roles are fulfilled by ER-targeted proteins such as LACS1 and LACS8 that may functionally overlap with LACS9.
Cutin and epidermal waxes
Plants contain many structural features that allow the representative species to grow and reproduce in a multitude of environmental niches, each adapting to the unique stresses, biotic and abiotic, present in each environment. One of these is the cuticle, the waxy layer that covers most of the above-ground surfaces of terrestrial plants (reviewed here). The cuticle is made up of cutin, a lipophilic layer containing a heavily cross-linked network of hydroxylated fatty acids and structurally related derivatives, and cuticular waxes (a complex mixture of very-long-chain fatty acids, primary and secondary alcohols, ketones, esters and other fatty acid-derived metabolites), which are deposited on and within the cutin layer. The cuticle is very important to proper plant growth and function; and as such was an extremely interesting topic for research. For many years, though, detailed analysis of cuticle synthesis was hampered by the complex nature of the chemical structure of the cuticle, and the physical intractability of the cutin layer itself. Chemical analysis of the component parts of cutin and cuticular waxes strongly indicated that both are synthesized from activated forms (likely CoA esters, in most cases) of long- and very-long-chain fatty acids. Therefore, the LACS enzymes and other members of the AAE superfamily were obvious targets for study. Creation and analysis of comprehensive sets of loss-of-function mutants greatly facilitated investigations of various steps of the cutin and cuticular wax biosynthetic pathways.
LACS1, -2, and -3 appear to be involved in biosynthesis of cutin and epidermal waxes. Various studies of lacs2 knockout mutants have shown that loss of this gene causes thinning of the abaxial cutin layers (Schnurr et al., 2004), defective seed set and seedling germination (Kurdyukov et al., 2006, and references therein), altered sensitivity to fungal and bacterial pathogens (Bessire et al., 2007, and references therein) and altered responses to osmotic stress (Deak and Malamy, 2005; MacGregor et al., 2008). All of these phenotypes are consistent with a role for LACS2 in production of cutin. The various physiological studies cited here are supported by biochemical studies in which LACS2 enzyme showed high activity towards 16-hydroxypalmitate, a cutin monomer precursor (Schnurr et al., 2004).
Given the closely linked nature of the cutin and wax layers in the cuticle, many laboratories have investigated the factors that influence cuticular wax components as well. While not yet completely understood, it is well established that oleic acid, present as oleoyl-CoA in the cytosol of plant cells, serves as the primer for synthesis for the complex pool of very-long-chain lipids (typically C26-C30) that make up the wax complement. The ultimate composition of the wax layer is determined by the contributions of two separate branches of the pathway. Analyses of loss-of-function mutants of LACS1 and LACS2 (both separately and combined) revealed key roles for both of these proteins in production of the normal amounts and composition of wax lipids (Lü et al., 2009; Weng et al., 2010). lacs1lacs2 double mutants in particular also showed several phenotypic defects due to altered wax biosynthesis (in addition to the underlying cutin defects already described for lacs2 above) including reduced fertility, poor seed germination, increased sensitivity to drought stress, and abnormal flower development (Lü et al., 2009; Weng et al., 2010).
LACS3 (along with LACS1 and LACS2) is strongly expressed in young stem epidermal tissues (Suh et al., 2005), and LACS3 is also a highly expressed gene in citrus fruit epidermis (Matas et al., 2010). These data suggest conserved roles for this group of proteins across most aerial plant species. Pulsifer et al. (2012) expressed these three proteins in mutant forms of yeast that lacked various components of the fatty acid import and activation pathways. These authors proposed that LACS1-3 may play dual roles in cuticle production, through both acyl-CoA synthesis and fatty acid transport (Pulsifer et al., 2012). Recent comprehensive reviews cover many aspects of this topic in greater detail (Kunst and Samuels, 2009; Shockey and Browse, 2011, and references therein).
Fatty acid β-oxidation
Fatty acids are a highly reduced form of carbon, and as such, are rich in energy. Most of this energy is captured as ATP synthesis through acetyl-CoA oxidation, via the tricarboxylic acid cycle in mitochondria. The peroxisomal enzymes of β-oxidation catabolize fatty acids to generate this supply of acetyl-CoA. LACS enzymes play a critical role in activating fatty acids released by lipases from stored lipids (primarily triacylglycerols) present in germinating seeds and other tissues where rapid lipid mobilization occurs.
Of the nine member LACS enzyme subfamily in Arabidopsis, both LACS6 and LACS7 are targeted to peroxisomes in plant cells (Fulda et al., 2002). The gene expression profiles of both genes are tightly correlated to the rate of usage of stored TAGs in germinating seedlings (Fulda et al., 2002; 2004) and both enzymes show high activity against seed-specific fatty acids (Shockey et al., 2002), suggesting that these two enzymes are directly involved in the peroxisomal β-oxidation pathway. Mutant analysis confirmed this hypothesis; lacs6lacs7 double mutants were compromised in their ability to utilize stored lipids and germinate properly (Fulda et al., 2004), similar to other β-oxidation mutants.
However, in contrast to other β-oxidation mutants, the lacs6lacs7 double mutant is not resistant to the effects of either 2,4-DB (a herbicide precursor), or indole-3-butryic acid (a plant hormone precursor), both of which are shortened by two carbon units by β-oxidation to their biologically active forms. β-Oxidation is also required to convert 3-oxo-2(29[Z]-pentenyl)-cyclopentane-1-octanoic acid (OPC-8:0) to jasmonic acid, a plant hormone with roles in both defense signaling and development. Blockages in this pathways lead to male sterility in Arabidopsis (Stintzi and Browse, 2000) and tomato (Li et al., 2005). The lacs6lacs7 double mutant is fertile, indicating that neither of these enzymes is responsible for CoA activation of OPC:8; proving that other AAE proteins are responsible for the activation of OPC:8, IBA, and 2,4-DB. Other recent studies have identified the enzymes that initiate β-oxidation of some of these alternative substrates. Wiszniewski et al. (2009) conducted a screen for 2,4-DB resistant mutants of Arabidopsis, and identified peroxisomally targeted AtAAE18 as a component of a β-oxidation pathway that acts upon 2,4-DB, while two groups (Koo et al., 2006; Kienow et al., 2008) identified At1g20510. This peroxisomal enzyme is related to the 4-coumarate ligase-like proteins of Arabidopsis AAE superfamily clade IV. The enzyme encodes OPC-8:0 CoA ligase activity. Knockout mutants overaccumulated OPC-8:0 at the expense of jasmonic acid, and the gene is upregulated in response to wounding or infection (Koo et al., 2006; Kienow et al., 2008).
The Other AAEs: The Rest of the Story? How Many More Stories Are There?
At the time of the first analysis of the superfamily in Arabidopsis (Shockey et al., 2003), only preliminary analyses had been conducted on a limited number of genes. As detailed in the previous sections, the past 9 years have seen the development of a much deeper biochemical understanding of many long-established cellular pathways, and of the roles played by AAE enzymes that participate in them. Equally exciting, though, are the numerous studies published since 2003 that have begun to clarify AAE functions that had only been hinted at in the past, or in some cases, not recognized at all:
-
A striking observation from the initial characterization of the Arabidopsis AAE superfamily was the extremely high number of genes that were expressed (most quite strongly) in flowers. Floral tissues produce various types of complex lipids, many of which are necessary for normal fertilization (Ma, 2005). Lipid metabolism in the tryphine, or pollen coat, is particularly important, as these lipids help to establish proper contact between pollen and stigma, through which pollen tube growth occurs. Wang and Li (2009) identified two LACS4-like cDNA clones in anthers of cotton. Both genes were strongly expressed during early pollen development. Gene silencing led to decreased LACS enzyme activity and acyl-CoA pool sizes, and male sterility (Wang and Li, 2009). Recently, Jessen et al. (2011) showed that the coordinated activities of LACS1 and LACS4 are necessary for production of both surface waxes and tryphine lipids. ACOS5 (At1g62940) also contributes essential components of pollen wall lipids. In vitro assays reveal ACOS5 as a medium- to long-chain fatty acyl-CoA synthetase (de Azevedo-Sauza et al., 2009). In tapetal cells of pollen, the products of ACOS5 are metabolized to complex ketides used in sporopollenin monomer biosynthesis (Kim et al., 2010; Grienenberger et al., 2010).
-
AAE activity has also been implicated in the biosynthesis of glucosinolates; a large class of secondary metabolites involved in pathogen and insect defense responses in cruciferous plants. As part of a large screen for Arabidopsis mutants altered in seed glucosinolate metabolism, BZO1 (At1g65880) was identified and characterized as a benzoic acid CoA ligase (Kliebenstein et al., 2007). This enzyme is required for the accumulation of benzoyloxyglucosinolates, and may act as a useful tool to study the pathway for synthesis of benzoic acid from phenylalanine, which is very poorly understood.
-
Phylloquinone is an aromatic ketone that functions as an electron acceptor during photosynthesis, forming part of the electron transport chain of Photosystem I. Proper phylloquinone synthesis is essential in plants. Kim et al. (2008) identified AtAAE14 (At1g30520) as o-succinylbenzoyl-coenzyme A (OSB-CoA) ligase, a previously uncharacterized intermediary step in the biosynthetic pathway from chorismate to phylloquinone.
-
Malonyl-CoA is the precursor for fatty acid synthesis and elongation and biosynthesis of flavonoids and phytoalexins. Malonyl-CoA is thought primarily to be produced from acetyl-CoA by acetyl-CoA carboxylase. However, previous studies in plants, animals, and microbes have suggested that malonyl-CoA may also be made directly from malonic acid by malonyl-CoA synthetase. Chen et al. (2011) identified AtAAE13 (At3g16170) and the human homolog Acyl-CoA Synthetase Family Member 3 (ACSF3) as malonyl-CoA synthetases. While the role of ACSF3 in humans and other mammals is not known, AAE13 appears to be essential for normal growth and reproduction in plants (Chen et al., 2011). Malonic acid is a competitive inhibitor of succinate dehydrogenase, a key enzyme of the tricarboxylic acid cycle, and thus it is likely that AAE13 activity acts as a detoxification mechanism by preventing buildup of high levels of malonic acid.
Morphological Complexity and AAE Gene Number Have Increased in Parallel as Plants Evolved
The evolutionary march from the relatively simple genetic makeup of prehistoric algae to the highly diverged and specialized families of sessile land-based plant species of today required the creation and specialization of an incredible number of new biological pathways. As described in more detail below, recent evidence shows that various AAE genes have evolved to assist in many of these roles. We were intrigued by the question of how the size and complexity of the AAE gene superfamily evolved as plants made the transition from water to land, and diversified once on land. The explosion of new technologies that enable fast and cheap genome sequencing has enabled comparison of genome size and complexity across various branches of the evolutionary tree, and has helped to reveal the major and minor genetic events that have driven the formation of different plant families. AAE gene superfamilies of three evolutionarily distant plant species were recently analyzed (Shockey and Browse, 2011). The results showed that the respective AAE gene families expanded and diversified as land plants evolved, but not in entirely predictable ways. Both the total number of and specific types of AAE genes that were created, maintained and expanded upon varied widely between these plant lineages.
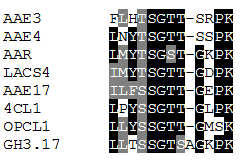
Currently, Arabidopsis is thought to contain 64 AAE genes (Shockey and Browse, 2011), including a gene encoding a putative aminoadipate semialdehyde dehydrogenase (AAR, EC 1.2.1.31; Torruella et al., 2009), a class of lysine metabolic enzymes not previously described in higher plants. The alignment of this motif from the putative AAR protein, and representatives from each of the seven clades, including the variant motif present in the hormone conjugase enzymes of clade III is shown in Figure 4. These enzymes couple plant hormones and amino acids, via an amide linkage rather than a CoA-derived thioester bond (Staswick et al., 2002).
Figure 4. Alignment of conserved AMP-binding motif (PROSITE PS00455:
[LIVMFY]-X-X-[STG]-[STAG]-G-[ST]-[STEI]-[SG]-X-[PASLIVM]-[KR]) from the putative AAR protein (described above) and representative proteins from the seven clades of the Arabidopsis AAE superfamily. This motif is the unifying feature for this family of genes.
The unicellular alga C. reinhardtii has a genome of comparable size to Arabidopsis, yet contains only 14 AAE genes, perhaps reflecting in part the simpler unicellular body plan and aquatic environment of this organism. The AAE gene family of C. reinhardtii is noteworthy due the lack of clade III-type hormone amino acid conjugases and the proliferation of acetyl-CoA synthetase-related genes (at least four of such, compared to only one of this type in Arabidopsis), in keeping with recent studies that have shown that C. reinhardtii contains a very broad repertoire of enzymes for the metabolism of acetate, pyruvate, and ethanol (Atteia et al. 2006). This alga also contains a type I polyketide synthase (PKS) gene. PKS was long thought to be exclusive to bacteria and fungi, but has recently been identified in several chlorophyte protists (John et al., 2008).
Physcomitrella patens is a bryophyte moss species whose genome (500 Mb) is approximately four times larger than that of Arabidopsis, yet contains only slightly more than half as many (37) AAE genes. Unlike C. reinhardtii, the AAE superfamily of P. patens does contain genes for likely hormone-amino acid conjugases, consistent with the presence of relatively simple auxin signaling mechanisms in this organism (Paponov et al., 2009). Also interesting is the presence of three LACS9 orthologs. It is not known whether all three actually are targeted to plastids in P. patens cells, but the absence of LACS9-type genes in algae compared to Arabidopsis and other land-based species suggests that the evolution of LACS9 (and its recruitment to the plastid proteome) could be one of the hallmarks that differentiates these two plant lineages.
The black cottonwood tree (Populus trichocharpa) contains the largest AAE superfamily of the plant species surveyed, with at least 77 genes, a twofold increase in genetic complexity despite a genome size nearly equal to that of P. patens (Tuskan et al., 2006). Evolution of the complete core group of long chain acyl-CoA synthetases also seems to have occurred during the evolution of vascular plants; LACS1, LACS2, and LACS8 are all present in the angiosperms but not in algae or bryophytes. The genomes of Arabidopsis and P. trichocharpa have undergone more duplication events, which is evident in their substantially higher numbers of clade III and clade VI genes, relative to C. reinhardtii and P. patens. P. trichocharpa contains a putative aminoadipate semialdehyde dehydrogenase (Torruella et al., 2009) that awaits detailed investigation to determine its true function. Black cottonwood also contains three AAE genes that are unique to the species set sampled in our recent work, but are conserved in several other monocot and dicot plant species. The biochemical functions and physiological roles of these four proteins are not known.
For comparison's sake, the human genome was recently analyzed for the presence of LACS and related genes using highly conserved amino acid sequence motifs. Watkins et al. (2007) detected at least 26 proven or likely human acyl-CoA synthetase genes. This set of genes likely makes up the majority of the AAE superfamily in humans. At approximately 3 billion bp, the human genome is much larger than any of the plant species described here, but contains much longer introns and intergenic regions than most plant genomes, thus making relative comparisons of AAE superfamily size and genome size a bit problematic. Nonetheless, it is clear that the unique metabolic demand placed on plants (compared to mammals) has helped drive the expansion of the AAE superfamily in this branch of the tree of life. As in plants, the biological function of many of the 26 ACS genes is not yet known, and will require detailed molecular analysis. Such findings, combined with the relative difficulty that still remains in the prediction of acyl substrate specificity and the intriguing gaps in our understanding of the biochemical roles of many of these enzymes, clearly indicate that many novel and exciting aspects of metabolism in plants and mammals still await discovery and investigation.
References
- Atteia, A., van Lis, R., Gelius-Dietrich, G., Adrait, A., Garin, J., Joyard, J., Rolland, N. and Martin, W. Pyruvate formate-lyase and a novel route of eukaryotic ATP synthesis in Chlamydomonas mitochondria. J. Biol. Chem., 15, 9909-9918 (2006) (DOI: 10.1074/jbc.M507862200).
- Bessire, M., Chassot, C., Jacquat, A.-C., Humphry, M., Borel, S., Petétot, J.M.-C., Métraux, J.-P. and Nawrath, C. A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. EMBO J., 26, 2158–2168 (2007) (DOI: 10.1038/sj.emboj.7601658).
- Chen, H., Kim, K.U., Weng, H. and Browse, J. Malonyl-coA synthetase, encoded by ACYL ACTIVATING ENZYME13, is essential for growth and development of Arabidopsis. Plant Cell, 23, 2247-2262 (2011) (DOI: 10.1105/tpc.111.086140).
- de Azevedo Souza, C., Barbazuk, B., Ralph, S.G., Bohlmann, J., Hamberger, B. and Douglas, C.J. Genome-wide analysis of a land plant-specific acyl:coenzymeA synthetase (ACS) gene family in Arabidopsis, poplar, rice and Physcomitrella. New Phytol., 179, 987–1003 (2008) (DOI: 10.1111/j.1469-8137.2008.02534.x).
- de Azevedo Souza, C., Kim, S.S., Koch, S. et al.A novel fatty acyl-coA synthetase is required for pollen development and sporopollenin biosynthesis in Arabidopsis. Plant Cell, 21, 507–525 (2009) (DOI: 10.1105/tpc.108.062513).
- Deak, K.I. and Malamy, J. Osmotic regulation of root system architecture. Plant J., 43, 17–28 (2005) (DOI: 10.1111/j.1365-313X.2005.02425.x).
- Dunkley, T.P.J., Hester, S., Shadforth, I.P., Runions, J., Weimar, T., Hanton, S.L., Griffin, J.L., Bessant, C., Brandizzi, F., Hawes, C., Watson, R.B., Dupree, P. and Lilley, K.S. Mapping the Arabidopsis organelle proteome. Proc. Natl Acad. Sci. U.S.A., 103, 6518–6523 (2006) (DOI: 10.1073/pnas.0506958103).
- Fulda, M., Heinz, E. and Wolter, F.P. Brassica napus cDNAs encoding fatty acyl-CoA synthetase. Plant Mol. Biol. 33, 911–922 (1997) (DOI: 10.1023/A:1005780529307).
- Fulda, M., Schnurr, J., Abbadi, A., Heinz, E. and Browse, J. Peroxisomal acyl-CoA synthetase activity is essential for seedling development in Arabidopsis thaliana. Plant Cell, 16, 394–405 (2004) (DOI: 10.1105/tpc.019646).
- Fulda, M., Shockey, J., Werber, M., Wolter, F.P. and Heinz, E. Two long-chain acyl-CoA synthetases from Arabidopsis thaliana involved in peroxisomal fatty acid β-oxidation. Plant J., 32, 93–103 (2002) (DOI: 10.1046/j.1365-313X.2002.01405.x).
- Grienenberger, E., Kim, S.S., Lallemand, B., Geoffroy, P., Heintz, C., de Azevedo Souza, C., Heitz, T., Douglas, C.J. and Legrand, M. Analysis of TETRAKETIDE α-PYRONE REDUCTASE function in Arabidopsis thaliana reveals a previously unknown, but conserved, biochemical pathway in sporopollenin monomer biosynthesis. Plant Cell, 22, 4067–4083 (2010) (DOI: 10.1105/tpc.110.080036).
- Gulick, A.M. Conformational dynamics in the acyl-CoA synthetases, adenylation domains of non-ribosomal peptide synthetases, and firefly luciferase. ACS Chem. Biol., 4, 811–827 (2009) (DOI: 10.1021/cb900156h).
- Hu, Y., Gai, Y., Yin, L., Wang, X., Feng, C., Feng, L., Li, D., Jiang, X.N. and Wang, D.C. Crystal structures of a Populus tomentosa 4-coumarate:CoA ligase shed light on its enzymatic mechanisms. Plant Cell, 22, 3093–3104 (2010) (DOI: 10.1105/tpc.109.072652).
- Jessen, D., Olbrich, A., Knüfer, J., Krüger, A., Hoppert, M., Polle, A. and Fulda, M. Combined activity of LACS1 and LACS4 is required for proper pollen coat formation in Arabidopsis. Plant J., 68, 715-726 (2011) (DOI: 10.1111/j.1365-313X.2011.04722.x).
- John, U., Beszteri, B., Derelle, E., Van de Peer, Y., Read, B., Moreau, H. and Cembella, A. Novel insights into evolution of protistan polyketide synthases through phylogenomic analysis. Protist, 159, 21–30 (2008) (DOI: 10.1016/j.protis.2007.08.001).
- Khurana, P., Gokhale, R.S. and Mohanty, D. Genome scale prediction of substrate specificity for acyl adenylate superfamily of enzymes based on active site residue profiles. BMC Bioinformatics, 11, 57–74 (2010) (DOI: 10.1186/1471-2105-11-57).
- Kienow, L., Schneider, K., Bartsch, M., Stuible, H.–P., Weng, H., Miersch, O., Wasternack, C. and Kombrink, E. Jasmonates meet fatty acids: functional analysis of a new acyl-coenzyme A synthetase family from Arabidopsis thaliana. J. Exp. Bot., 59, 403–419 (2008) (DOI: 10.1093/jxb/erm325).
- Kim, H.U., van Oostende, C., Basset, G.J.C. and Browse, J. The AAE14 gene encodes the Arabidopsis o-succinylbenzoyl-CoA ligase that is essential for phylloquinone synthesis and photosystem-I function. Plant J., 54, 272–283 (2008) (DOI: 10.1111/j.1365-313X.2008.03416.x).
- Kliebenstein, D.J., D’Auria, J.C., Behere, A.S., Kim, J.H., Gunderson, K.L., Breen, J.N., Lee, G., Gershenzon, J., Last, R.L. and Jander, G. Characterization of seed-specific benzoyloxyglucosinolate mutations in Arabidopsis thaliana. Plant J., 51, 1062–1076 (2007) (DOI: 10.1111/j.1365-313X.2007.03205.x).
- Koo, A.J.K., Chung, H.S., Kobayashi, Y. and Howe, G.A. Identification of a peroxisomal acyl-activating enzyme involved in the biosynthesis of jasmonic acid in Arabidopsis. J. Biol. Chem., 281, 33511–33520 (2006) (DOI: 10.1074/jbc.M607854200).
- Kunst, L. and Samuels, L. Plant cuticles shine: advances in wax biosynthesis and export. Curr. Opin. Plant Biol., 12, 721–727 (2009) (DOI: 10.1016/j.pbi.2009.09.009).
- Kurdyukov, S., Faust, A., Nawrath, C., Bär, S., Voisin, D., Efremova, N., Franke, R., Schreiber, L., Saedler, H., Métraux, J.P. and Yephremov, A. The epidermis specific extracellular BODYGUARD controls cuticle development and morphogenesis in Arabidopsis. Plant Cell, 18, 321–339 (2006) (DOI: 10.1105/tpc.105.036079).
- Li, C., Schilmiller, A.L., Liu, G., Lee, G.I., Jayanty, S., Sageman, C., Vrebalov, J., Giovannoni, J.J., Yagi, K., Kobayashi, Y. and Howe, G.A. Role of β-oxidation in jasmonate biosynthesis and systemic wound signaling in tomato. Plant Cell., 17, 971-986 (2005) (DOI: 10.1105/tpc.104.029108).
- Lü, S., Song, T., Kosma, D.K., Parsons, E.P., Rowland, O. and Jenks, M.A. Arabidopsis CER8 encodes LONG-CHAIN ACYL-COA SYNTHETASE 1 (LACS1) that has overlapping functions with LACS2 in plant wax and cutin synthesis. Plant J., 59, 553–564 (2009) (DOI: 10.1111/j.1365-313X.2009.03892.x).
- Ma, H. Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu. Rev. Plant Biol., 56, 393–434 (2005) (DOI: 10.1146/annurev.arplant.55.031903.141717).
- MacGregor, D.R., Deak, K.I., Ingram, P.I. and Malamy, J. Root system architecture in Arabidopsis grown in culture is regulated by sucrose uptake in the aerial tissues. Plant Cell, 20, 2643–2660 (2008) (DOI: 10.1105/tpc.107.055475).
- Matas, A.J., Agustí, J., Tadeo, F.R., Talón, M. and Rose, J.K.C. Tissue-specific transcriptome profiling of the citrus fruit epidermis and subepidermis using laser capture microdissection. J. Exp. Bot., 61, 3321–3330 (2010) (DOI: 10.1093/jxb/erq153).
- Paponov, I.A., Teale, W., Lang, D., Paponov, M., Reski, R., Rensing, S.A. and Palme, K.The evolution of nuclear auxin signalling. BMC Evol. Biol., 9, 126 (2009) (DOI: 10.1186/1471-2148-9-126).
- Pulsifer, I.P., Kluge, S. and Rowland, O. Arabidopsis long-chain acyl-CoA synthetase 1 (LACS1), LACS2, and LACS3 facilitate fatty acid uptake in yeast. Plant Physiol. Biochem., 51, 31-39 (2012) (DOI: 10.1016/j.plaphy.2011.10.003).
- Schmelz, S. and Naismith, J.H. Adenylate-forming enzymes. Curr. Opin. Struct. Biol., 19, 666-671 (2009) (DOI: 10.1016/j.sbi.2009.09.004).
- Schnurr, J., Shockey, J. and Browse, J. The acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis. Plant Cell, 16, 629–642 (2004) (DOI: 10.1105/tpc.017608).
- Schnurr, J.A., Shockey, J.M., De Boer, G.J. and Browse, J.A. Fatty acid export from the chloroplast: molecular characterization of a major plastidial acyl-coenzyme A synthetase from Arabidopsis. Plant Physiol., 129, 1700–1709 (2002) (DOI: 10.1104/pp.003251).
- Shah, M.B., Ingram-Smith, C., Cooper, L.L., Qu, J., Meng, Y., Smith, K.S. and Gulick, A.M. The 2.1Å crystal structure of an acyl-CoA synthetase from Methanosarcina acetivorans reveals an alternate acyl-binding pocket for small branched acyl substrates. Proteins, 77, 685-698 (2009) (DOI: 10.1002/prot.22482).
- Shockey, J. and Browse J. Genome-level and biochemical diversity of the acyl-activating enzyme superfamily in plants. Plant J., 66, 143–160 (2011) (DOI: 10.1111/j.1365-313X.2011.04512.x).
- Shockey, J.M., Fulda, M.S. and Browse, J. Arabidopsis contains a large superfamily of acyl-activating enzymes. Phylogenetic and biochemical analysis reveals a new class of acyl-coenzyme A synthetases. Plant Physiol., 132, 1065–1076 (2003) (DOI: 10.1104/pp.103.020552).
- Shockey, J.M., Fulda, M.S. and Browse, J.A. Arabidopsis contains nine long-chain acyl-coenzyme A synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol., 129, 1710–1722 (2002) (DOI: 10.1104/pp.003269).
- Staswick, P.E., Tiryaki, I. and Rowe, M.L. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell, 14, 1405–1415 (2002) (DOI: 10.1105/tpc.000885).
- Stintzi, A., and Browse, J. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc. Natl. Acad. Sci. U.S.A., 97, 10625-1030 (2000) (DOI: 10.1073/pnas.190264497).
- Suh, M.C., Samuels, A.L., Jetter, R., Kunst, L., Pollard, M., Ohlrogge, J. and Beisson, F. Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis. Plant Physiol., 139, 1649–1665 (2005) (DOI: 10.1104/pp.105.070805).
- Torruella, G., Suga, H., Riutort, M., Peretó, J. and Ruiz-Trillo, I. The evolutionary history of lysine biosynthesis pathways within eukaryotes. J. Mol. Evol., 69, 240–248 (2009) (DOI: 10.1007/s00239-009-9266-x).
- Tuskan, G.A., DiFazio, S., Jansson, S. et al. The genome of black cottonwood Populus trichocharpa (Torr. and Gray). Science, 313, 1596–1604 (2006) (DOI: 10.1126/science.1128691).
- Wang, X.-L. and Li, X.-B. The GhACS1 gene encodes an acyl-CoA synthetase which is essential for normal microsporogenesis in early anther development of cotton. Plant J., 57, 473–486 (2009) (DOI: 10.1111/j.1365-313X.2008.03700.x).
- Watkins, P.A., Maiguel, D., Jia, Z. and Pevsner. J. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J Lipid Res., 12, 2736-2750 (2007) (DOI: 10.1194/jlr.M700378-JLR200).
- Weng, H., Molina, I., Shockey, J. and Browse, J. Organ fusion and defective cuticle function in a lacs1 lacs2 double mutant of Arabidopsis. Planta, 231, 1089–1100 (2010) (DOI: 10.1007/s00425-010-1110-4).
- Wiszniewski, A.A.G., Zhou, W., Smith, S.M. and Russell, J.D. Identification of two Arabidopsis genes encoding a peroxisomal oxidoreductase-like protein and an acyl-CoA synthetase-like protein that are required for responses to pro-auxins. Plant Mol. Biol., 69, 503–515 (2009) (DOI: 10.1007/s11103-008-9431-4).
- Zhao, L., Katavic, V., Li, F., Haughn, G.W. and Kunst, L. Insertional mutant analysis reveals that long-chain acyl-CoA synthetase 1 (LACS1), but not LACS8, functionally overlaps with LACS9 in Arabidopsis seed oil biosynthesis. Plant J., 64, 1048–1058 (2010) (DOI: 10.1111/j.1365-313X.2010.04396.x).
In This Section
- Plant Fatty Acid Synthesis
- Production of Unusual Fatty Acids in Plants
- Arabidopsis Acyl-Coenzyme A-Binding Proteins
- Long Chain acyl-coA Synthetases and Other Acyl Activating Enzymes
- Plant Triacylglycerol Synthesis
- Triacylglycerol Biosynthesis in Eukaryotic Microalgae
- Subcellular Oil Droplets and Oleosins in Plants
- Triacylglycerol Mobilisation in Plants
- Role of Transcription Factors in Storage Lipid Accumulation in Plants
- Biosynthesis of Plant Lipid Polyesters
- Rubber Biosynthesis in Plants
- Carotenoid Biosynthesis and Regulation in Plants
- The Oxylipin Biosynthetic Pathways in Plants
- N-Acylphosphatidylethanolamines (NAPEs), N-acylethanolamines (NAEs) and Other Acylamides: Metabolism, Occurrence and Functions in Plants
- Phosphoinositide Signaling in Plants
- Plant Lipidomics
- 50 years of Galactolipid Research: The Beginnings
- Transport and function of lipids in the plant phloem