Biosynthesis of Plant Lipid Polyesters
The author: Isabel Molina, School of Life Sciences and the Environment, Algoma University, Sault Ste. Marie, Ontario, P6A 2G4, Canada
All higher plants depend on extracellular lipophilic barriers that control the movement of water, solutes, and gases, and that provide an interface with the environment. Cutin and suberin are two distinct types of insoluble plant polyesters of fatty acids and glycerol that constitute the matrices of such barriers. Cutin constitutes the structural component of the cuticle, the protective and waterproofing film that covers the aerial surface of primary aerial plant organs and represents one of the largest biological interfaces. Suberin is constitutively produced by various internal and external cells during development, and is also induced to form a barrier in response to stresses from the environment and wounding. Although both cutin and suberin have structural similarities and analogous functions in the protection of plant organs, they are distinguished by their deposition sites and chemical composition.
Because of their structural complexity and insolubility, progress in understanding the structure, enzymology and genetics of lipid polyesters has been slow. However, significant progress has been made in the last decade with the availability of molecular genetic tools developed for the model plant Arabidopsis thaliana. The following sections summarize our current understanding of the structure and biosynthesis of the lipid polyesters cutin and suberin, and provide some background on their functions and chemistries. Other lipid polyesters found on the surface of stigmas or pollen grains, and the biosynthesis of cuticular waxes and suberin-associated polyaromatics are not covered here.
The Cuticle and Cutin
Occurrence and functions
Vascular plants and some bryophytes have developed a cuticle, an outer hydrophobic skin that protects them from desiccation and acts as a mechanical barrier against pathogens, insects, and UV radiation. The cuticle also controls nonstomatal gas exchange. The phenotypes of certain cuticular mutants suggest that it also plays a role in maintaining the separation of organs during organogenesis. The cuticle covers the external surface of the epidermal cell walls of leaves, primary stems, flowers, and fruits. Internal cuticles also occur; these have been described within seed coats and lining the substomatal cavity.
Structure and chemical composition
The major hydrophobic constituents of the plant cuticle are the insoluble polymers, cutin and cutan, and a mixture of chloroform-soluble epicuticular and intracuticular lipids, collectively called waxes (Fig. 1). The cuticle usually has an amorphous appearance when observed by electron microscopy, but in some plants it presents a lamellar ultrastructure.
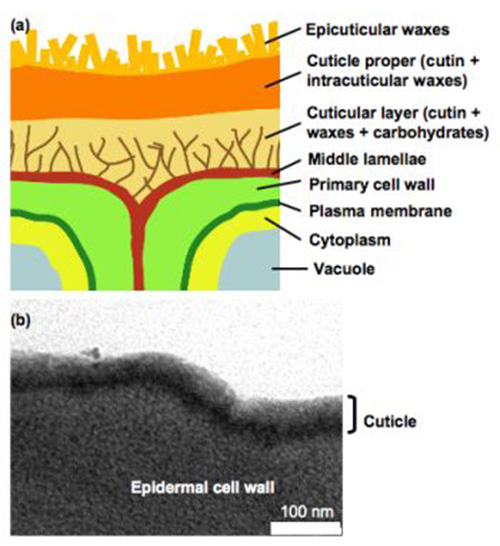
Figure 1. Structure of the plant cuticle. (a) Diagrammatic structure of epidermis and cuticle. (b) Ultrastructure of the cuticle of the leaf epidermis of Arabidopsis plants.
Cuticles have several layers, which are defined on the basis of their position and chemical composition: the cuticular layer, attached to the cell wall, which contains polymers, waxes, and polysaccharides that extend from the underlying cell wall; the cuticle proper, comprised of cutin and intracuticular waxes; and epicuticular waxes covering the cuticle proper. The thickness of these layers and their composition depend on the species, anatomical location and developmental stage. The overall cuticular thickness ranges from 0.1 to 14 μm in leaves, and contains <20 to 600 μg of cutin per cm2 surface area. In fruits the cutin content can reach 1.5 mg/cm2.
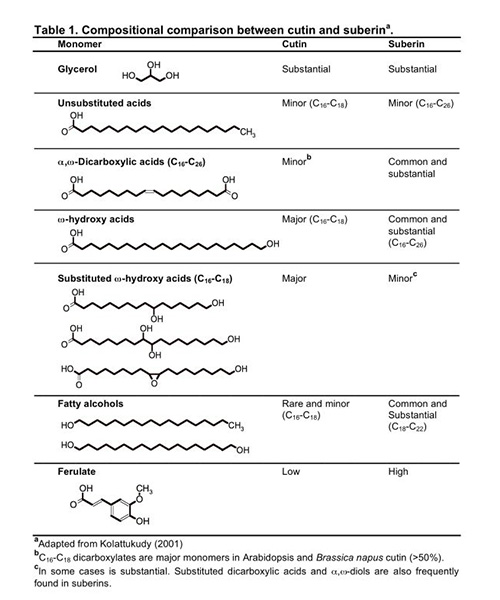
Cutin is a polyester of C16 and C18 oxygenated fatty acids and glycerol. Monomers released after chemical cleavage of ester bonds usually include ω-hydroxy and ω-hydroxy-epoxy fatty acids, which are derived from C16 saturated and C18 unsaturated fatty acids (Table 1). Minor amounts of hydroxycinnamic acids have been also reported as structural components. Although dicarboxylic acids are typically found in suberin, these structures are major monomers found in Arabidopsis thaliana and Brassica napus cuticles. However, the most abundant monomer in these species is C18:2 dicarboxylic acid, which is usually found only in trace amounts in suberins. Cutan is another structural polymer that remains as a nondepolymerizable fraction after ester-bond hydrolysis of cutin; it is possibly composed of cutin monomers mostly linked by ether and C-C bonds. Cuticular waxes include very-long-chain alkanes and substituted derivatives such as fatty acids, primary and secondary alcohols, aldehydes and ketones.
Cutin monomers are esterified to each other by their primary hydroxyl groups (Fig. 2) forming a linear polyester. In addition, esters formed between the carboxyl group of one fatty acid and a hydroxyl group of glycerol or a secondary hydroxyl group of another fatty acid allow a branched structure such as cross-linked and dendrimeric arrangements. However, the three-dimensional structure of cutin remains unresolved. It is also unclear if the insolubility of the polyester is a consequence of covalent linkage to the cell wall or cutan, or of the high molecular weight of the polymer.
Figure 2. A hypothetical arrangement of monomers that could be found in a ω-hydroxy fatty acid-rich cutin. Primary ester bonds are dominant, with some secondary ester bonds from mid-chain hydroxylated monomers. R: aliphatic polyester.
Suberized Cell Walls and Suberin
Occurrence and functions
While the cuticle lies on the outer face of the primary cell wall, suberin is located on the inside of the primary cell wall, usually close to the plasma membrane (Fig. 3). Plants synthesize suberin to create a hydrophobic barrier to water and solute diffusion through cell walls during normal development, or to provide a barrier in response to environmental stresses. A familiar example of suberized tissue is cork, constituted by the outer bark cells (periderm) of cork oak (Quercus suber). Suberin is also deposited in periderm walls of underground organs (i.e. tubers, roots), and in the root endodermis. A material with composition intermediate between suberin and cutin is often deposited surrounding the bundle sheaths of monocot leaves, perhaps to prevent CO2 released during decarboxylation reactions from diffusing out of the bundle sheath cells. In mature seeds, suberin has been found in seed coat layers, and sealing off the chalazal region of the inner seed coat after disconnection from vascular tissue. Exposure to cold, mineral stress and fungal infection are some examples where suberization occurs as a response to external factors. It is also deposited as a wound response by injured plant cells, even in those cells that normally synthesize cutin.
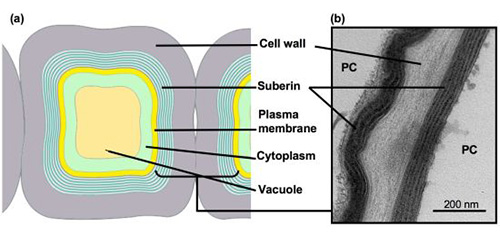
Figure 3. Schematic (a) and transmission electron microscopic (b) views of Arabidopsis suberized root cells.
Suberized peridermal cells present the typical lamellar deposition within the cell walls. PC, peridermal cell.
Chemical composition and structure
Suberin is a heteropolymer, consisting of an aliphatic polyester associated with cross-linked polyaromatics and embedded waxes. Upon transesterification of suberin, the monomers released include C16-C28 ω-hydroxy fatty acids and C16-C26 α,ω-dicarboxylic acids, the latter of which are diagnostic monomers, unsubstituted very-long-chain fatty acids (VLCFAs; >C18) and alcohols, glycerol and ferulate (Table 1). Unlike cuticular waxes, suberin-associated waxes in part resemble the structural constituents of the polyester and seem to be biosynthetically related. Suberin waxes include alkanes, primary alcohols, fatty acids, alkyl ferulates and monoacylglycerols. The aromatic network of suberin is a polymer of C-C and ether-linked hydroxycinnamic acids, N-feruloyltyramine and monolignols, and therefore these monomers are not released by polyester depolymerization methods.
Because suberin is an insoluble heteropolymer deposited within the plant cell wall, its structure has been difficult to study and remains unclear. Suberin frequently presents a lamellar ultrastructure when observed by transmission electron microscopy (TEM), with alternating light and dark bands (Fig. 3). Models to explain the macromolecular structure of suberin are based on chemical analyses and ultrastructural data of cork or potato periderm. In these models, the aliphatic monomers are linearly arranged, forming the electron-translucent bands. The nature of the opaque bands is less understood; these may correspond to glycerol, esterified phenolics, and possibly waxes. Ferulic acid has been proposed to provide a covalent link between cell-wall-embedded polyaromatics and the suberin polyester. However, the phenotypes of recently characterized Arabidopsis knockout mutants question this last hypothesis, as well as the presence of an extended aliphatic polyester.
Biosynthesis
Biosynthesis of cutin and suberin
Suberin contains those monomers typically found in cutin, namely, fatty acids and ω-hydroxylated monomers, and also alcohols, α,ω-dicarboxylic acids, and ferulate that are considered diagnostic of suberin (Table 1). Textbooks and reviews usually describe the synthesis of cutin and suberin aliphatic polyesters as distinct pathways, with chain elongation and conversion of ω-hydroxy acids to dicarboxylic acids being specific for suberin monomers. However, data from Arabidopsis has shown that dicarboxylates are not exclusively found in suberin, and that the same gene families seem to be involved in the synthesis of both types of polyesters. Hence, monomers with chain lengths beyond C20, higher levels of aromatics, and primary alcohol synthesis can be considered as specifically associated with suberin. Here, the current knowledge on the synthesis and assembly of both cutin and suberin aliphatics is summarized together.
Early radiolabeling experiments performed by Kolattukudy’s group 30 years agogave insight into the pathways involved in oxidation of C16 and C18 monomers, using cutinizing (i.e. extracts of bean leaf epidermis) and suberizing (i.e. wound-healed potato slices) tissues. Since enzymes involved in biosynthesis of cutin/suberin monomers are possibly associated to membranes and/or are part of enzyme complexes, biochemical approaches to isolate these proteins have not been successful. Several recombinant plant fatty acid ω-hydroxylases were shown to be active in yeast expression systems, but these experiments could not demonstrate their involvement in cutin or suberin. Thus, genetic approaches have been particularly helpful to dissect biosynthetic pathways. Several cutin mutants have been identified in part because they resemble transgenic plants that overexpress fungal cutinase (cutinases are enzymes that hydrolyze the ester linkages), which show alterations in the structure of the cuticle and organ fusions, or because they present increased susceptibility to pathogens. Further development of quantitative analyses of lipid polyester monomers in Arabidopsis allowed the use of reverse genetic approaches to discover new genes involved in lipid polyester synthesis. Identification of candidate genes has been aided by published transcriptome analyses from Arabidopsis epidermis and cork tree epidermis, as well as public repositories for microarray data. Reverse genetics has also been recently applied to demonstrate the function of suberin-synthesizing genes in potato periderm.
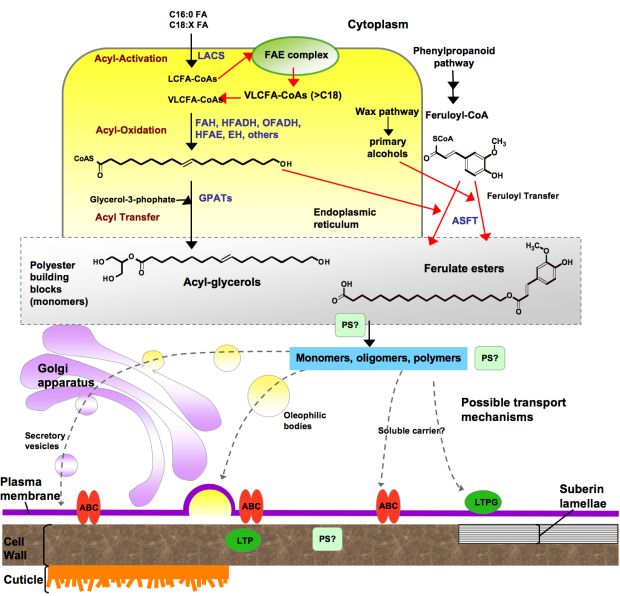
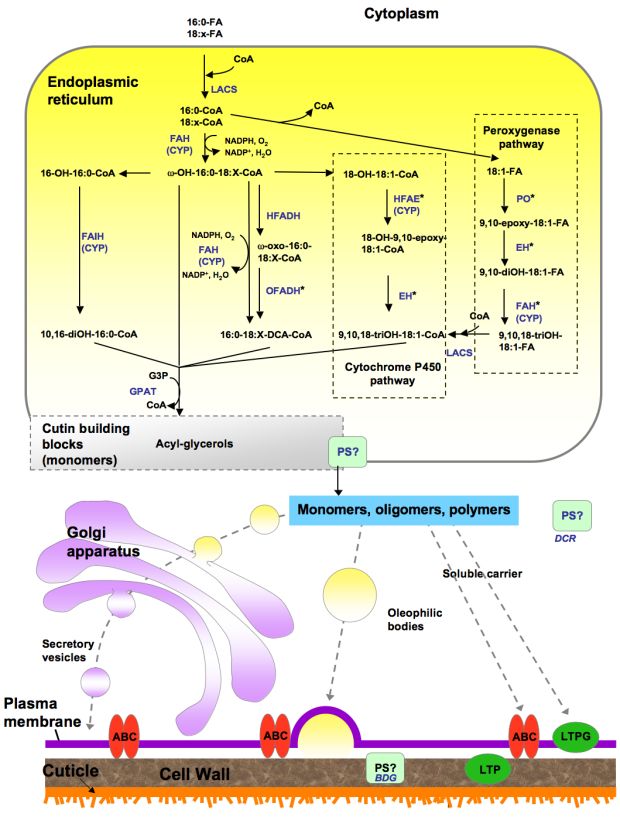
The acyl precursors for lipid polyesters are common C16 and C18 cellular fatty acids. In suberin, some of these acyl precursors are elongated by enzymes of the fatty acid elongation (FAE) complex to produce very-long-chain acyl-CoAs. Fatty acyl chains undergo a series of modification reactions, namely, activation to coenzyme A thioesters, oxidation and esterification to a glycerol-based acceptor to produce acyl glycerols, which may constitute lipid polyester building blocks. Because in most cases the in vivo substrates for the biosynthetic enzymes are unknown, there is uncertainty regarding the order of such reactions. Figure 4 represents an overview of major steps in lipid polyester synthesis, where one of the possible orders is shown. Subcellular expression of reporter protein fusions and membrane targeting predictions suggest that monomer biosynthesis takes place at the endoplasmic reticulum (ER). More detailed pathways are provided in Figure 5 (cutin synthesis) and Figure 6 (aliphatic suberin synthesis). All of the pathways presented herein are based primarily on Arabidopsis data, and the genes involved are shown in more detail at the web site http://aralip.plantbiology.msu.edu/pathways.
Figure 4. Overview of possible steps involved in lipid polyester synthesis.
The scheme represents one possible scenario for the synthesis of lipid polyester building blocks (possibly acylglycerols and fatty acids), where acyl oxidation occurs after elongation and before esterification to glycerol-3-phosphate. Fatty acid-modification and acyl glycerol synthesis steps are thought to take place in the ER. Transport mechanisms required to transfer monomers or oligomers/polymers to the site of polyester assembly remain hypothetical. Polyester synthase(s), required to link monomers to produce high-molecular-weight polyesters, as well as polymerization site(s) remain unknown. Only a few structures of possible intermediates are depicted to illustrate major steps. Red arrows indicate reactions that apply only to suberin. (Adapted from Pollard et al., 2008.)
Abbreviations: ABC, ATP binding cassette transporter; ASFT, aliphatic suberin feruloyl transferase; EH, epoxide hydrolase; FAH, Fatty acyl ω-hydroxylase; GPAT, glycerol 3-phosphate acyltransferase; HFADH, ω-hydroxy fatty acyl dehydrogenase; HFAE, ω-hydroxy fatty acyl epoxygenase; LACS, long-chain acyl-coA synthetase; LCFA, long-chain fatty acid; LTP, lipid transfer proteins; LTPG, glycosylphosphatidyl-inositol (GPI)-anchored protein; OFADH, ω-oxo fatty acyl dehydrogenase; PS, polyester synthase; VLCFA, very-long-chain fatty acid. CoA,
Figure 5. Proposed pathways for the synthesis of cutin.
This biosynthetic scheme represents one possible order of reactions for the synthesis of cutin building blocks (possibly acyl glycerols). Acyl chains are activated to coenzyme-A by LACS, acyl-CoAs are oxidized and then esterified to glycerol-3-phosphate by GPAT enzymes. The site of polymerization and, thus, the nature of the molecules transported remain unknown. Only ABC transporters have been associated with cutin synthesis; a membrane-bound LTP (LTPG1) is involved in cuticular wax export, and similar proteins may have a role in cutin synthesis. Other possible transport mechanisms include oleophilic bodies, secretory vesicles and PM-associated ER domains (not shown in the figure). Apoplastic movement of monomers or oligomers might be facilitated by LTPs. Polyester synthase(s), required to link monomers to produce high molecular weight polyesters, remain unknown, although an extracellular lipase (BDG) has been proposed as a cutin synthase, and an acyltransferase (DCR) has been suggested to form cutin oligomers in the cytoplasm. Known or putative enzymes are indicated in blue. (Adapted from Pollard et al., 2008). *Gene/enzymes catalyzing these steps have not been identified. Abbreviations: ABC, ATP binding cassette transporter; BDG, BODYGUARD; CYP, cytochrome P450 monooxygenase; DCR, DEFECTIVE IN CUTICULAR RIDGES; EH, epoxide hydrolase; FAH, Fatty acyl ω-hydroxylase; FAIH, Fatty acyl in-chain hydroxylase; G3P, glycerol-3-phosphate; GPAT, glycerol 3-phosphate acyltransferase; HFADH, ω-hydroxy fatty acyl dehydrogenase; HFAE, ω-hydroxy fatty acyl epoxygenase; LACS, long-chain acyl-coA synthetase; LTP, lipid transfer proteins; LTPG, glycosylphosphatidyl-inositol (GPI)-anchored protein; OFADH, ω-oxo fatty acyl dehydrogenase; PO, peroxygenase; PM, plasma membrane; PS, polyester synthase. NADPH, NADP+ DCA ER
Figure 6. Proposed pathways for the synthesis of suberin aliphatic polyester.
This biosynthetic scheme represents one possible order of reactions for the synthesis of suberin building blocks (i.e., acyl glycerols), where acyl chains are activated to coenzyme-A by LACS, acyl-CoAs are oxidized and then esterified to glycerol-3-phosphate by GPAT enzymes. Fatty acid activation, acyl-oxidation and acyl glycerol synthesis are believed to take place in the endoplasmic reticulum. The site of polymerization and, thus, the nature of the molecules transported remain unknown. Only an ABC transporter has been associated with suberin synthesis; a membrane-bound LTP (LTPG1) has been associated with cuticular wax export, and similar proteins could be involved in suberin assembly. Polyester synthase(s), required to link monomers to produce high molecular weight polyesters, remain unknown. Known or putative enzymes are indicated in blue. (Adapted from Pollard et al., 2008.)
*Gene/enzymes catalyzing these steps have not been identified.
Abbreviations: 4CL, 4-coumarate ligase; ABC, ATP binding cassette transporter; ASFT, aliphatic suberin feruloyl transferase; CCoAOMT, caffeoyl CoA O-methyltransferase; CYP, cytochrome P450 monooxygenase; DCA, dicarboxylic acid; ECR, enoyl-CoA reductase; FAE, fatty acid elongation; FAH, fatty acyl ω-hydroxylase; FAR, alcohol-forming fatty acyl-CoA reductase; G3P, glycerol-3-phosphate; GPAT, glycerol 3-phosphate acyltransferase; HCD, β–hydroxyacyl-CoA dehydratase; HFADH, ω-hydroxy fatty acyl dehydrogenase; KCR β–ketoacyl-CoA reductase; KCS, β-ketoacyl-CoA synthase; LACS, long-chain acyl-coA synthase; LTP, lipid transport protein; LTPG, glycosylphosphatidyl-inositol (GPI)-anchored protein; OFADH, ω-oxo fatty acyl dehydrogenase; OHFA, ω-hydroxy fatty acid; PS, polyester synthase. HACD VLCFA DCAcyl
Acyl elongation
In suberin, VLCFAs are often major components. The ER-associated fatty acid elongation (FAE) complex is responsible for the chain extension of C16 and C18 acyl chains to VLCFAcyl-CoAs. In each four-step reaction, the FAE complex extends the acyl chains by two carbons (Fig. 6). The first reaction is catalyzed by a β-ketoacyl-CoA synthase (KCS), the rate-limiting enzyme in the complex. Arabidopsis contains 21 KCS genes and, because saturated long chains are common to several biosynthetic pathways (suberin, waxes, and sphingolipids), these enzymes can act in more than one metabolic route, thus complicating any attempt to assign a specific function to individual isoforms. Nevertheless, at least one Arabidopsis gene, AtKCS2, has been shown to be involved in root suberin monomer elongation. Similarly, StKC6 has been shown to catalyze the elongation of monomers with chain lengths of C28 and higher in potato periderm.
Acyl oxidation
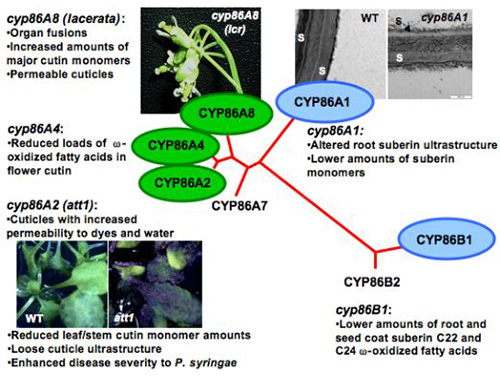
Hydroxylated fatty acids are major constituents of plant lipid polyesters. In plants, hydroxylation of the terminal methyl of aliphatics (ω-position) is catalyzed by cytochrome-P450-dependent (CYP) enzymes, most of them belonging to the CYP86 and CYP94 subfamilies. Their functions have been biochemically demonstrated by heterologous expression in yeast and insect cells. These enzymes use molecular oxygen to produce the functionalized substrate and a molecule of water. However, evidence for their role in cutin or suberin biosynthesis was only provided later by forward or reverse genetics strategies (Fig. 7). The Arabidopsis att1 mutants present thinner, more permeable cuticles that have reduced loads of ω-oxidized cutin monomers, thus providing evidence that ATT1 (encoding CYP86A2) is functionally implicated in cutin biosynthesis in planta. Consistent with its role, the ATT1 promoter directs YFP reporter expression to cutin-synthesizing tissues (Fig. 8). The lacerata (lcr) mutant (cyp86a8) has a phenotype compatible with a defective cuticle but, unlike other characterized knockout mutants of this subfamily, leaf cutin accumulated higher levels of C18:2 ω-oxidized monomers. Although Arabidopsis leaf and stem cutin has an unusual composition rich in unsaturated dicarboxylic acids, its flowers present a classical cutin composition with high content of 10,16-dihydoxy fatty acid. CYP86A4 is involved in ω-hydroxylation forming 16-hydroxy fatty acid, and CYP77A6 catalyzes the in-chain hydroxylation of this monomer to produce 10,16-dihydoxy fatty acid.
Figure 7. The phenotypes of Arabidopsis mutants revealed the role of cytochromes P450 enzymes in lipid polyester synthesis.
Three genes of the CYP86 subfamily of P450-omega oxidases are related to cutin synthesis (green), and two are involved in suberin synthesis (blue). Att1, aberrant induction of type three genes mutant; lcr: lacerata mutant; S, suberin; WT, wild-type.
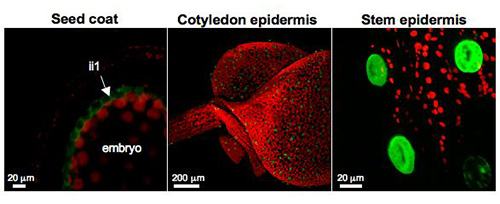
Figure 8. CYP86A2 (ATT1) promoter is active in cutin-synthesizing tissues
Confocal laser scanning microscope (CLSM) images of Arabidopsis transgenic plants expressing promoterCYP86A2::YFP fusions showed ATT1 promoter activity in the inner integument1 (ii1) layer of developing seed coats (left), cotyledon epidermis (center), and stem epidermis (right).
At least two other members of the CYP86 subfamily participate in suberin synthesis. CYP86A1 catalyzes root suberin saturated and unsaturated <C20 monomer oxidation and is expressed in tissues where suberization takes place (Fig. 9); the potato CYP86A1-homologous gene plays a similar role in potato periderm. CYP86B1 is involved in C22 and C24 fatty acid ω-oxidation in Arabidopsis root and seed coat suberin.
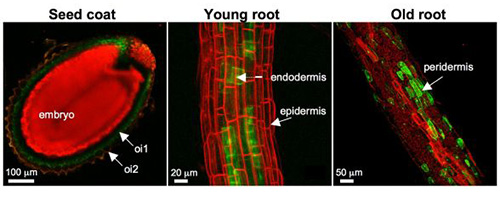
Figure 9. CYP86A1 promoter is active in suberizing tissues
Confocal laser scanning microscope (CLSM) examination of transgenic Arabidopsis lines expressing promoterCYP86A1::YFP fusions revealed that the promoter is active in outer integument1 (oi1) layer of developing seed coats (left), endodermis of young roots (center), and peridermis of old roots (right) that underwent secondary thickening.
Two different pathways for the synthesis of dicarboxylic acids have been proposed (Fig. 5). One postulates that CYP multifunctional enzymes are able to catalyze the complete set of reactions leading to the oxidation of a terminal methyl group to the carboxyl function, similar to tobacco CYP94A5 or Arabidopsis CYP94C1. The second route involves two subsequent dehydrogenases that oxidize ω-hydroxy fatty acids to ω-oxo fatty acids and then to α,ω-dicarboxylic acids. This was first supported by in vitro labeling experiments, and later by genetic evidence shown in Arabidopsis cutin. It is proposed that HOTHEAD (HTH) encodes an ω-hydroxyacid-dehydrogenase that converts ω-hydroxy fatty acids to ω-oxo fatty acids. However, the second enzyme remains unknown.
In cutins of many species, C18 hydroxy-epoxy acids and trihydroxy acids are major constituents. Labeling experiments performed in cutin-synthesizing epidermal tissues suggested that CYP enzymes are involved in both ω-hydroxylation and epoxidation of C18 cutin monomers, and that further hydrolytic cleavage by an epoxide hydrolase produces the 9,10,18-trihydroxy C18:1 acid. A different pathway, involving peroxygenase and epoxide hydrolase activities, along with a CYP ω-hydroxylase, was later found in soybean seedlings. In this system, the soybean peroxygenase preferred oleic acid rather than the hydroxylated monomer as substrate. Furthermore, the soluble epoxide hydrolase purified from soybean seedlings preferred the nonhydroxylated epoxide monomer. Hence, the CYP activity was required downstream of these steps (Fig. 5). The involvement of the peroxygenase pathway in cutin biosynthesis was later supported by in planta inhibition of this enzyme, which resulted in a thinner cuticle and increased susceptibility of maize leaves to infection by fungi. Thus far, the specific genes/enzymes involved in these pathways have not been identified.
Acyl activation
Members of the family of long-chain acyl-CoA synthetases (LACS) are also required for polyester biosynthesis. Knockout mutants of LACS2 present a defective cuticle with reduced monomer loads, providing evidence that intermediates in cutin synthesis need to be activated by LACS2. In vitro assays demonstrated that this enzyme can use both fatty acids and ω-hydroxylated fatty acids as substrates, and it is therefore unclear where in the pathway these activation steps take place in vivo. In addition, LACS1 has been recently identified as an acyl-CoA synthetase that preferentially activates C16 fatty acids in cutin synthesis. Cuticles of lacs1lacs2 double knockout plants are more permeable and have lower cutin amounts than those of either parent, implying that LACS1 and LACS2 have overlapping functions in cutin synthesis.
Acyl esterification
Since glycerol has an important function in cross-linking both cutin and suberin monomers, an acyltransferase activity is needed to form acylglycerols. Several genes of the Arabidopsis GPAT (glycerol-3-phosphate acyltransferase) family have been characterized by gain- and loss-of-function approaches, and are now known to have a role in lipid polyester synthesis. GPAT5 encodes an enzyme involved in the transfer of aliphatics (C20-C24) to a glycerol-based acceptor in Arabidopsis seed coat and root suberin. In leaf and stem, the functionally redundant GPAT4 and GPAT8 genes were shown to be required for the deposition of the major cutin monomers. GPAT6 encodes an acyltransferase that preferentially uses C16 aliphatics as substrates for the synthesis of flower cutin. Unlike the glycerol-3-phosphate acyltransferases that catalyse the synthesis of plant membrane and storage lipids, the enzymes involved in cutin and suberin transfer acyl groups to the sn-2 rather than sn-1 position.
Glycerol esterified to aliphatics and to hydroxycinnamic acids, and ferulic acid esters of very-long-chain fatty alcohols have been found in suberin waxes. Such structures may represent intermediates that could be further incorporated to the suberin polymer by a peroxidase, accounting for polyester insolubility. Furthermore, ferulate esterified to glycerol and to ω-hydroxy fatty acids was released by partial depolymerization. Feruloyl-transferase activity was first observed in extracts from potato tubers and tobacco cell suspensions, but no enzymes responsible for the hydroxycinnamoyl-transferase reaction were identified in those works. Recently, genetic and biochemical studies have shown that an enzyme of the BAHD family of acyltransferases functions as an aliphatic suberin feruloyl-transferase (ASFT) in Arabidopsis seed coat and root suberin and potato periderm. ASFT catalyzes the acyl-transfer from feruloyl-CoA to ω-hydroxy fatty acids and fatty alcohols. Although asft mutants contain only 5% of the wild-type ester-linked ferulate in suberin, the polyester still remains insoluble. Thus, this phenotype questions the hypothesis that esterified ferulate is a major linker between the aliphatic and aromatic domains.
Transport processes
Little is known about how lipid polyester precursors, which may possibly include fatty acids, acylglycerols, or oligomeric esters, are transported to the polyester deposition site. Oleophilic body- and/or vesicle-mediated transport has been suggested based on ultrastructural analysis of rapidly expanding rice internodes. Other possible mechanisms include ATP-binding cassette (ABC) transporters and lipid transfer proteins (LTPs). Indeed, a knockout mutant of the WHITE-BROWN COMPLEX 11 (WBC11) gene encoding a plasma membrane-localized ABC transporter has been shown to have reduced loads of cutin and root suberin, suggesting that this mechanism could be involved in transport of polyester intermediates. LTPs are small, soluble extracellular proteins that could mediate the transport of fatty acids or acylglycerols through the hydrophilic cell walls to the site of polymerization, although there is not direct evidence for such role. However, mutant analyses suggested that a glycosylphosphatidylinositol (GPI)-anchored LTP (LTPG1) might function in transport of cuticular lipids in Arabidopsis epidermis. For both WBC11 and LTPG1, it remains unknown how their lipid substrates reach the plasma membrane from their synthesis site.
Polyester assembly
The enzyme responsible for the assembly of cutin monomers into a polymer (i.e. polyester synthase), and whether the polymerization reaction takes place within the cell or in the apoplast remain enigmatic. Because fungal lipases can polymerize suberin monomers in vitro, lipases have been proposed to behave as bifunctional enzymes. Using a genetic approach, an epidermis-specific lipolytic enzyme localized to the cell wall, the BODYGUARD (BDG) gene product, has been suggested to act as an extracellular synthase required for Arabidopsis cutin polymerization. Furthermore, an enzyme that belongs to the family of GDSL-motif putative lipases has been identified in epidermal cells of Agave americana leaves and has been suggested to be involved in both hydrolysis and transfer of activated monomers in cutin synthesis. In addition to lipases, at least one member of the BAHD family of acyltransferases, encoded by the DEFECTIVE IN CUTICULAR RIDGES (DCR) gene, has been proposed to function in polymer assembly. No biochemical activity has been confirmed for any of these potential polyester synthases.
Regulation of polyester synthesis
Lipid polyesters are synthesized during normal development and in response to various stress conditions, in coordination with wax and, mainly in suberin, phenylpropanoid biosynthetic pathways. Consequently, these highly orchestrated processes need tight regulation. The transcription factor WIN1/SHN1, a member of the AP2-domain/ethylene response element binding protein super family, has been shown to induce expression of several genes of cutin biosynthesis. In addition, AtMYB41 has been identified as a regulator of cuticle deposition and cell expansion that is only expressed under stress conditions. The regulatory mechanisms that control the deposition of suberin are unknown. It is clear that the biosynthetic machinery responds to environmental stimuli, and that the regular pattern observed in the suberin lamellae must result from a specific mechanism that has not yet been elucidated. Candidate genes for cork regulation include members of the R2R3 MYB, APETALA1 and WRKY families.
Recommended Resources
A web site that includes details on the genes of Arabidopsis cutin and suberin biosynthesis and the lipid polyester composition of various Arabidopsis tissues is available at: http://aralip.plantbiology.msu.edu/pathways.
Methods of analysis of cutin and suberin, with a focus on Arabidopsis are presented in Li-Beisson et al. (2010).
- Beisson, F., Li, Y., Bonaventure, G., Pollard, M. and Ohlrogge. J. The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell, 19, 351-368 (2007).
- Bernards, M. Demystifying suberin. Can. J. Bot., 80, 227-240 (2002).
- Bonaventure, G., Beisson, F., Ohlrogge, J. and Pollard, M. Analysis of the aliphatic monomer composition of polyesters associated with Arabidopsis epidermis: occurrence of octadeca-cis-6,cis-9-diene-1,18-dioate as the major component. Plant J., 40, 920-930 (2004).
- Franke, R. and Schreiber, L. Suberin - a biopolyester forming apoplastic plant interfaces. Curr. Opin. Plant Biol., 10, 252-259 (2007).
- Franke, R., Briesen, I., Wojciechowski, T., Faust, A., Yephremov, A., Nawrath, C. and Schreiber, L. Apoplastic polyesters in Arabidopsis surface tissues - A typical suberin and a particular cutin. Phytochemistry, 66, 2643-2658 (2005).
- Graça, J. and Santos, S. Suberin: a biopolyester of plants' skin. Macromol. Biosci., 7, 128-135 (2007).
- Kandel, S., Sauveplane, V., Olry, A., Diss, L., Benveniste, I. and Pinot, F. Cytochrome P450-dependent fatty acid hydroxylases in plants. Phytochem. Rev., 5, 359-372 (2006).
- Kannangara, R., Branigan, C., Liu, Y., Penfield, T., Rao, V., Mouille, G., Hofte, H., Pauly, M., Riechmann, J.L. and Broun, P. The transcription factor WIN1/SHN1 regulates cutin biosynthesis in Arabidopsis thaliana. Plant Cell, 19, 1278-1294 (2007).
- Kolattukudy, P.E. Polyesters in higher plants. Adv. Biochem. Eng. Biotechnol., 71, 1-49 (2001).
- Li, Y., Beisson, F., Koo, A.J.K., Molina, M., Pollard, M. and Ohlrogge, J. Identification of acyltransferases required for cutin synthesis and production of cutin with suberin-like monomers. Proc. Natl. Acad. Sci. U.S.A., 104, 18339-18344 (2007).
- Li-Beisson, Y., Pollard, M., Sauveplane, V., Pinot, F., Ohlrogge, J. and Beisson, F. Nanoridges that characterize the surface morphology of flowers require the synthesis of cutin polyester. Proc. Natl. Acad. Sci. U.S.A., 106, 22008-22013 (2009).
- Li-Beisson, Y., Shorrosh, B., Beisson, F., Andersson, M.X., Arondel, V., Bates, P.D., Baud, S., Bird, D., DeBono, A., Durrett, T.P., Franke, R.B., Graham, I.A., Katayama, K., Kelly, A.A., Larson, T., Markham, J.E., Miquel, M., Molina, I., Nishida, I., Rowland, O., Samuels, L., Schmid, K.M., Wada, H., Welti, R., Xu, C., Zallot, R. and Ohlrogge, J. Acyl-lipid Metabolism. In: The Arabidopsis Book, (American Society of Plant Biologists, Rockville, MD) (2010).
- Molina, I., Bonaventure, G., Ohlrogge, J.and Pollard, M. The lipid polyester composition of Arabidopsis thaliana and Brassica napus seeds. Phytochemistry, 67, 2597-2610 (2006).
- Molina, I., Li-Beisson, Y., Beisson, F., Ohlrogge, J.B. and Pollard, M. Identification of an Arabidopsis feruloyl-coenzyme A transferase required for suberin synthesis. Plant Physiol., 151, 1317-1328 (2009).
- Nawrath, C. The biopolymers cutin and suberin. In: The Arabidopsis Book, pp. 1-14 (American Society of Plant Biologists, Rockville, MD - www.bioone.org/doi/abs/10.1199/tab.0021) (2002).
- Pollard, M., Beisson, F., Li, Y. and Ohlrogge, J.B. Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci., 13, 236-246 (2008).
- Soler, M., Serra, O., Molinas, M., Huguet, G., Fluch, S. and Figueras, M. A genomic approach to suberin biosynthesis and cork differentiation. Plant Physiol., 144, 419-431 (2007).
- Suh, M.C., Samuels, A.L., Jetter, R., Kunst, L., Pollard, M., Ohlrogge, J. and Beisson. F. Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis. Plant Physiol., 139, 1649-1665 (2005).
In This Section
- Plant Fatty Acid Synthesis
- Production of Unusual Fatty Acids in Plants
- Arabidopsis Acyl-Coenzyme A-Binding Proteins
- Long Chain acyl-coA Synthetases and Other Acyl Activating Enzymes
- Plant Triacylglycerol Synthesis
- Triacylglycerol Biosynthesis in Eukaryotic Microalgae
- Subcellular Oil Droplets and Oleosins in Plants
- Triacylglycerol Mobilisation in Plants
- Role of Transcription Factors in Storage Lipid Accumulation in Plants
- Biosynthesis of Plant Lipid Polyesters
- Rubber Biosynthesis in Plants
- Carotenoid Biosynthesis and Regulation in Plants
- The Oxylipin Biosynthetic Pathways in Plants
- N-Acylphosphatidylethanolamines (NAPEs), N-acylethanolamines (NAEs) and Other Acylamides: Metabolism, Occurrence and Functions in Plants
- Phosphoinositide Signaling in Plants
- Plant Lipidomics
- 50 years of Galactolipid Research: The Beginnings
- Transport and function of lipids in the plant phloem