Phospholipases
The Authors: Michelle Bamji-Mirza and Zemin Yao, Department of Biochemistry, Microbiology and Immunology, University of Ottawa, 451 Smyth Road, Ottawa, Canada K1H 8M5. DOI: 10.21748/lipidlibrary.39190
I. Introduction
Phopholipases, as the term suggests, are a group of enzymes that catalyze the cleavage of phospholipids. While some phospholipases possess substrate specificity for certain phospholipid species, some can catalyze the cleavage of other lipophillic molecules (such as triacylglycerols) in addition to phospholipids. This review highlights the structural and functional diversity of phospholipases in their tissue distribution, subcellular localization, and substrate specificity, as well as the functional importance of the phospholipid metabolites. Phospholipids are not simply structural components of cellular membranes. Enzymatic processing of phospholipids by phospholipases converts these molecules into lipid mediators or second messengers (such as arachidonic acid, phosphatidate and diacylglycerol) that play key roles in membrane trafficking, signal transduction, cell proliferation and apoptosis. Thus, phospholipases have been implicated in lipid metabolism and disease progression. There has been a tremendous interest from the pharmaceutical industry in developing selective and potent inhibitors of these enzymes.
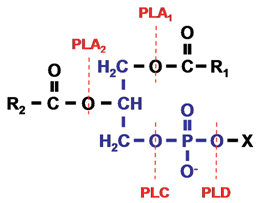
Phospholipids (or glycerophospholipids) consist of a glycerol-3-phosphate molecule esterified at its carbon 1 (sn-1) and carbon 2 (sn-2) positions to nonpolar fatty acids and at its phosphoryl group to a polar head group (a nitrogenous base, a glycerol, or an inositol unit), X (Fig. 1). Phospholipids are structurally complex as a result of various combinations of polar head groups and fatty acyl chains. Typically, the sn-1 position of phospholipids is occupied by saturated fatty acids, whereas the sn-2 position is occupied by unsaturated fatty acids. A comprehensive review on classification, structure and cellular functions of lipids has recently been published (Bou Khalil, M., et al., 2010).
Figure 1. Phospholipid structure and the site of action of phospholipases. The phospholipid molecule consists of a glycerol-3-phosphate (blue colour) esterified at its sn-1 and sn-2 positions to nonpolar fatty acids (R1 and R2, respectively) and at its phosphoryl group to a polar head group, X. Phospholipase A1 and phospholipase A2 cleave the acyl ester bonds at sn-1 and sn-2, respectively. Phospholipase C cleaves the glycerophosphate bond whereas phospholipase D removes the head group, X. PLA, phospholipase A; PLC, phospholipase C; PLD, phospholipase D.
On the basis of the ester bond that is cleaved within a phospholipid molecule, phospholipases are grouped into four families, namely A, B, C and D. Phospholipase A enzymes cleave the acyl ester bond at either the sn-1 (phospholipase A1) or sn-2 (phospholipase A2) position (Fig. 1). The term phospholipase B is given to phospholipases that hydrolyze acyl ester bonds at both sn-1 and sn-2 positions. Enzymes grouped under phospholipase C cleave the glycerophosphate bond, while phospholipase D enzymes remove the polar head group (Fig. 1). The phospholipase A, phospholipase C, and phospholipase D enzyme families are further classified into subgroups and the characteristics of these enzymes are described below.
II. Phospholipase A
Phospholipase A1
Phospholipase A1 enzymes hydrolyze the fatty acid at the sn-1 position of phospholipids. Isozymes of phospholipase A1 can be divided into two groups according to their cellular localization: group 1 consists of intracellular enzymes, while group 2 consists of extracellular enzymes. There are three members in the mammalian intracellular phospholipase A1 subfamily, namely phosphatidic acid-preferring phospholipase A1, p125, and KIAA0725p (Table 1). Phosphatidic acid-preferring phospholipase A1 is highly expressed in mature testis and is thought to participate in spermiogenesis. P125 binds to the COP-II component Sec23, and is involved in the organization of COP-II vesicles (which mediate vesicular transport from the endoplasmic reticulum to the Golgi). KIAA0725p is localized to the Golgi apparatus and is required for anterograde trafficking (vesicular transport from Golgi to the plasma membrane). A common structural feature of these enzymes is a lipase consensus sequence, Gly-x-Ser-x-Gly, containing the active site serine and the x denotes any amino acid.
|
Table 1. Identified human phospholipase A1 |
||||
| Group | Name | Phospholipase activity | Triacylglycerol hydrolase activity | Enzymatic or physiological Function |
|---|---|---|---|---|
| Intracellular | PA-PLA1 | phosphatidate specific | X | spermiogenesis |
| p125 | phosphatidate specific | ? | COPII vesicle organization | |
| KIA0725p | phosphatidate specific | X | membrane trafficking from Golgi | |
| Extracellular | PS-PLA1 | phosphatidate and lysophosphatidic acid specific | X | lysophosphatidic acid production |
| mPA-PLA1α | phosphatidate specific | X | lysophosphatidic acid production | |
| mPA-PLA1β | Phosphatidate specific | X | lysophosphatidic acid production | |
| pancreatic lipase | intestinal triacylglycerol hydrolysis | |||
| lipoprotein lipase | hydrolyze mainly triacylglycerol in plasma | |||
| hepatic lipase | hydrolyze triacylglycerol and phospholipids in plasma | |||
| endothelial lipase | hydrolyze mainly phospholipids in plasma | |||
| pancreatic lipase related protein-1 | ? | ? | regulate phospholipids in vivo | |
| pancreatic lipase related protein-2 | intestinal triacylglycerol hydrolysis | |||
| pancreatic lipase related protein-3 | ? | ? | ? | |
| mPA-PLA1, membrane-associated phosphatidic acid preferring phospholipase A1; PA-PLA1, phosphatidic acid specific phospholipase A1; PS-PLA1, phosphatidylserine selective phospholipase A1. | ||||
The mammalian extracellular phospholipase A1 enzymes include phosphatidylserine selective phospholipase A1, membrane-associated phosphatidic acid selective phospholipase A1α, membrane-associated phosphatidic acid selective phospholipase A1β, pancreatic lipase, lipoprotein lipase, hepatic lipase, endothelial lipase, and pancreatic lipase-related proteins-1, -2, and -3 (Table 1). All of the secreted phospholipase A1 enzymes belong to a gene family called the pancreatic lipase gene family (see Section V). Classically, members of this gene family are involved in the hydrolysis of dietary or endogenous triacylglycerol. Phosphatidylserine selective phospholipase A1, membrane-associated phosphatidic acid selective phospholipase A1α, and membrane-associated phosphatidic acid selective phospholipase A1β thus form a subfamily because they do not hydrolyze triacylglycerol. The hydrolytic activities of pancreatic lipase-related protein-1 and -3 are largely unknown.
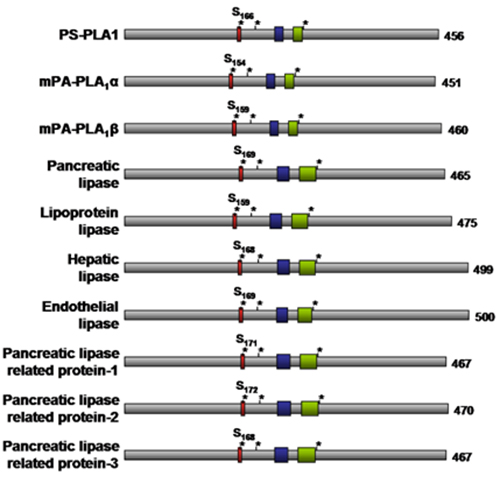
The suggested 3D structure for members of the pancreatic lipase gene family is modeled after the crystal structure of one of its members, pancreatic lipase. The gene family members share multiple conserved motifs, including the Gly-x-Ser-x-Gly lipase consensus sequence, a catalytic Ser-Asp-His triad, cysteine residues responsible for intramolecular disulfide bond formation, and lipid binding surface loops, including the lid domain and the β9 loop (Fig. 2). Structure-function analyses among family members revealed that the lipid binding surface loops (lid domain and β9 loop) affect lipid substrate specificity of the enzymes by restricting access to the catalytic site. Notably, extracellular phospholipase A1 members that exhibit triacylglycerol hydrolase activity have long lids (22-23 amino acids) and long β9 loops (18-19 amino acids), whereas the lids (7-12 amino acids) and β9 loops (12-13 amino acids) are shorter in phosphatidylserine selective phospholipase A1, membrane-associated phosphatidic acid selective phospholipase A1α, and membrane-associated phosphatidic acid selective phospholipase A1β (Fig. 2) that do not possess triacylglycerol hydrolysis activity (Table 1).
Figure 2. Schematic structure of extracellular phospholipase A1 enzymes. The red box denotes the lipase consensus sequence (Gly-x-Ser-x-Gly) with the active site serine (Serxxx) highlighted. The three asterisks denote the catalytic Ser-Asp-His triad. The blue box denotes the β9 loop whereas the green box denotes the lid domain. The number of amino acid residues for each enzyme is indicated to the right. PS-PLA1, phosphatidylserine selective phospholipase A1; mPA-PLA1, membrane-associated phosphatidic acid selective phospholipase A1.
The extracellular phospholipase A1 enzymes share extracellular location and sequence homology but their physiological roles differ vastly. Phosphatidylserine selective phospholipase A1 hydrolyzes phosphatidylserine and produces lysophosphatidylserine and fatty acid. Normally, phosphatidylserine is on the cytosolic side of the plasma membrane but through various stimuli phosphatidylserine can be exposed to the outer leaflet of the plasma membrane. During the blood coagulation cascade for instance, phosphatidylserine becomes externalized on the cell surface and regulates thrombin which is the central molecule in this pathway. Phosphatidylserine externalization is increased during the early stages of apoptosis and is the signal by which apoptotic cells are recognized and removed by phagocytes. Phosphatidylserine is also an enzyme co-factor for isoforms of protein kinase C (involved in diverse signal transduction pathways). Since phosphatidylserine selective phospholipase A1 acts on and thus regulates the levels of phosphatidylserine it too has been implicated in blood coagulation, apoptosis, and activation of protein kinase C among other functions. Interestingly, treatment of rats with lipopolysaccharide, to induce endotoxin shock, dramatically upregulated phosphatidylserine selective phospholipase A1 expression, suggesting phosphatidylserine selective phospholipase A1 has a role in pathological states.
The membrane-associated phosphatidic acid preferring phospholipase A1 isozymes α and β act on phosphatidic acid and produce lysophosphatidic acid, which also has multiple biological functions ranging from platelet aggregation and smooth muscle contraction to the stimulation of cell proliferation and motility. Thus, because the phospholipid substrates and/or metabolites of these phospholipase A1 hydrolytic reactions play integral roles in various pathways and signaling cascades, these enzymes are implicated in various biological functions.
Phospholipase A2
Phospholipase A2 enzymes hydrolyze phospholipids at the sn-2 position (Fig. 1). As mentioned, the sn-2 position of phospholipids contains unsaturated fatty acids such as arachidonic acid. Eicosanoids are a family of compounds (including prostaglandins and leukotrienes) that are produced by many cell types (such as macrophages) from arachidonic acid. There has been considerable pharmaceutical interest in characterizing phospholipase A2 enzymes owing to their role in the production of lipid mediators in inflammation, such as arachidonic acid and its eicosanoid derivatives prostaglandin and leukotriene. To date, 15 groups (I – XV) of phospholipase A2 have been identified and sorted into five main categories, namely, secreted phospholipase A2, Ca2+-dependent cytosolic phospholipase A2, Ca2+-independent phospholipase A2, platelet activating factor acetylhydrolase phospholipase A2, and lysosomal phospholipase A2 (Table 2). The first phospholipase A2 was initially discovered in snake venom, and later phospholipase A2 enzymes were found in other organisms including mammals (Table 2).
|
Table 2: Fifteen groups of phospholipase A2 |
|||
| Name (Group) | Type | Alternate name | Feature |
|---|---|---|---|
| IA | secreted PLA2 | N/A | 7 disulfide bonds |
| IB | N/A | 7 disulfide bonds | |
| IIA | N/A | 7 disulfide bonds | |
| IIB | N/A | 6 disulfide bonds | |
| IIC | N/A | 8 disulfide bonds | |
| IID | N/A | 7 disulfide bonds | |
| IIE | N/A | 7 disulfide bonds | |
| IF | N/A | 6 disulfide bonds | |
| III | N/A | 8 disulfide bonds | |
| IVA | Ca2+-dependent cytosolic PLA2 | cPLA2α | Ca2+-dependent phospholipid binding domain |
| IVB | cPLA2β | Ca2+-dependent phospholipid binding domain | |
| IVC | cPLA2γ | acylated | |
| IVD | cPLA2δ | Ca2+-dependent phospholipid binding domain | |
| IVE | cPLA2ε | Ca2+-dependent phospholipid binding domain | |
| IVF | cPLAyζ | Ca2+-dependent phospholipid binding domain | |
| V | secreted PLA2 | N/A | 6 disulfide bonds |
| VIA-1 | Ca2+ independent PLA2 | iPLA2 | 8 ankryin repeats |
| VIA-2 | iPLA2β | 7 ankryin repeats | |
| VIB | iPLA2γ | membrane bound, lysophospholipase activity |
|
| VIC | iPLA2δ | integral membrane protein, lysophospholipase activity |
|
| VID | iPLA2ε | acylglycerol transacylase; triacylglycerol hydrolase |
|
| VIE | iPLA2ζ | acylglycerol transacylase; triacylglycerol hydrolase |
|
| VIF | iPLA2η | acylglycerol transacylase; triacylglycerol lipase |
|
| VIIA | platelet activating factor acetylhydrolase (PAF-AH) | lipoprotein-associated PLA2 | secreted, α/β hydrolase |
| VIIB | PAF-AH II | intracellular, myristoylated, α/β hydrolase | |
| VIIIA | PAF-AH Ib (α1 subunit) | intracellular, Ser-His-Asp triad | |
| VIIIB | PAF-AH Ib (α2 subunit) | intracellular, Ser-His-Asp triad | |
| IX | secreted PLA2 | N/A | 6 disulfide bonds |
| X | N/A | 8 disulfide bonds | |
| XIA | N/A | 6 disulfide bonds | |
| XIB | N/A | 6 disulfide bonds | |
| XII | N/A | 7 disulfide bonds | |
| XIII | N/A | 0 disulfide bonds | |
| XIV | N/A | 2 disulfide bonds | |
| XV | Lysosomal PLA2 | N/A | Ser-His-Asp triad |
| PLA2, phospholipase A2; PAF-AH, platelet activating factor acetylhydrolase. | |||
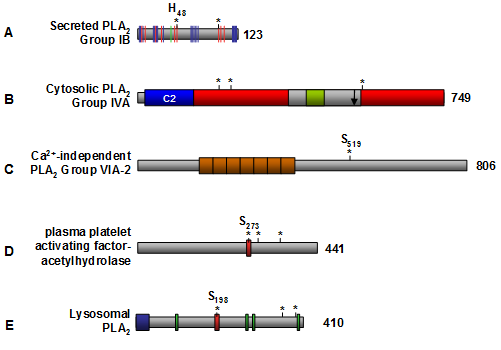
The secreted phospholipase A2 enzymes include Groups I through III, V, and IX through XIV phospholipase A2 (Table 2) and they share common features such as low molecular weight (13–15 kDa), a catalytic His-Asp dyad, Ca2+ bound in the active site, and conserved disulfide bonds (Fig. 3A). The molecular mechanisms of secreted phospholipase A2 action are not well understood. Many studies have examined the role that secreted phospholipase A2 play in eicosanoid release, but the results are inconclusive. Upregulation of Groups IIA, V, and X phospholipase A2 was associated with an increase in eicosanoids in a cytosolic phospholipase A2 (Group IVA)-dependent manner. However, a specific inhibitor of the Group IIA phospholipase A2 showed no therapeutic effects in human phase II clinical trials.
Figure 3. Schematic structure of phospholipase A2 enzymes. (A) Representative secreted phospholipase A2 isozyme, Group IB. The red bars indicate the essential amino acid residues within the active site (including the catalytic Hisxx-Asp dyad denoted by asterisks), the green bar denotes the Ca2+ binding site, and the blue bars indicate the interfacial binding surface residues that are in contact with phospholipid substrate. (B) Representative cytosolic phospholipase A2 isozyme, Group IVA. The Ca2+-dependent phospholipid binding domain (C2) is shown in blue, the α/β hydrolase catalytic fold is shown in red, the cap region is colored in grey, and the lid region is colored in green. The amino acid residues within the active site are denoted by asterisks, and the phosphatidylinositol 4,5-bisphosphate binding site is shown by an arrow. (C) The most studied Ca2+-independent phospholipase A2 is Group VIA-2. The orange boxes indicate the ankyrin repeats, and the Serxxx denotes the active site serine. (D) Structure of plasma platelet activating factor-acetylhydrolase. The red box denotes the lipase motif (Gly-x-Ser-x-Gly), the asterisks denote the catalytic Ser-Asp-His triad, and the active site serine (Serxxx) is highlighted. (E) Structure of lysosomal phospholipase A2. The blue box indicates the putative N-terminal signal sequence, the green boxes denote putative N-glycosylation sites, the red box denotes the lipase motif (Gly-x-Ser-x-Gly), and the asterisks denotes the catalytic Ser-Asp-His triad that contains Sxxx. The number of amino acid residues for each enzyme is indicated to the right. C2 domain, Ca2+-dependent phospholipid binding domain; PLA2, phospholipase A2.
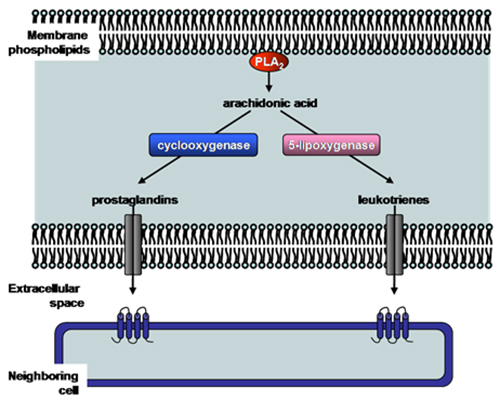
The Ca2+-dependent cytosolic phospholipase A2 enzymes, also known as Group IV phospholipase A2 (Table 2), contain a Ca2+-dependent phospholipid binding domain and a catalytic α/β hydrolase domain (Fig. 3B). Both of these domains are required for the cytosolic phospholipase A2 activity. The most characterized Group IVA phospholipase A2 also contains a cap region within which the lid domain presumably regulates substrate entrance into the active site (Fig. 3B). Group IVA phospholipase A2 is the only known phospholipase A2 with a preference for arachidonic acid in the sn-2 position, thus it represents the central enzyme that mediates the generation of eicosanoids (Fig. 4). It is presumably regulated by intracellular Ca2+ concentrations, its phosphorylation state and the binding of various activators. The available structural information of Group IVA phospholipase A2 has allowed the development of inhibitors. Two of the most promising drug candidates, indole derivative inhibitors such as efipladib (developed by Wyeth Research) and 2-oxoamide inhibitors (developed by Yaksh et al., 2006), have shown success in reducing inflammatory effects in animal models.
Figure 4. Production of eicosanoids by phospholipase A2 enzymes. The main function of certain phospholipase A2 enzymes (such as Group IV cytosolic phospholipase A2) is to produce eicosanoids (prostaglandins and leukotrienes). Phospholipase A2 hydrolyzes membrane phospholipids to release arachidonic acid. Cyclooxygenase or 5-lipoxygenase then converts arachidonic acid to either prostaglandins or leukotrienes, respectively. These short-lived molecules can be effluxed, and bind and activate specific receptors on neighboring cells to exert their biological effect such as pain and inflammation. PLA2, phospholipase A2.
The Ca2+-independent phospholipase A2 enzymes (also called Group VI enzymes (Table 2)) are cytosolic and do not require Ca2+ for activity. The most extensively studied Ca2+-independent phospholipase A2 is Group VIA-2 (also known as iPLA2β, PLA2G6, and PNPLA9). It has been implicated in many biological pathways; from maintaining basal cell membrane homeostasis by phospholipid remodeling to signal transduction in pancreatic β-cells leading to insulin release. A striking feature of Group VIA-2 phospholipase A2 is its lack of specificity for the fatty acid present at the sn-2 position of phospholipids, the enzyme being able to hydrolyze practically any fatty acid. A structural feature specific to Group VIA-2 phospholipase A2 is ankyrin repeats located within the amino-terminal half of the protein (Fig. 3C) which mediate protein-protein interactions. The Group VIA-2 phospholipase A2 sequence also contains several putative consensus sequences for caspase cleavage leading to the generation of Group VIA-2 phospholipase A2 fragments with increased biological activity. For example, Atsumi et al. (2000) showed that 2 by caspase-3. The loss of the amino-terminal region of Group VIA-2 phospholipase A2 resulted in a more active enzyme which accelerated membrane phospholipid destruction.
So far, little is known about Group VIA-1 phospholipase A2 and its cellular functioning. Another Ca2+-independent phospholipase A2 member, Group VIB phospholipase A2, exhibits phospholipase A2 and lysophospholipase activities but also has phospholipase A1 activity specific for palmitoyl-containing phosphatidylcholine. It is presumably membrane bound, but its cellular function is unclear. Group VIC phospholipase A2 (Table 2) is an integral membrane protein expressed in neurons of humans, and it possesses phospholipase A2 and lysophospholipase activities. Inhibition of Group VIC phospholipase A2 resulted in axonal degeneration; however, its physiological function has been elusive. Three Group VI phospholipase A2 enzymes (namely Group VID, VIE, and VIF phospholipase A2) (Table 2) have recently been identified and possess triacylglycerol hydrolase and acylglycerol transacylase activities. The physiological role of these enzymes remains to be defined.
The platelet activating factor acylhydrolase phospholipase A2 (Group VII and VIII phospholipase A2) (Table 2) catalyzes the hydrolysis of the sn-2 ester bond of platelet activating factor (acetyl-glyceryl-ether-phosphorylcholine) and related proinflammatory phospholipids. One platelet activating factor acylhydrolase (Group VIIA) is secreted, and thus is also referred to as plasma platelet activating factor acylhydrolase, or lipoprotein associated phospholipase A2 (Table 2). The other two PAF-AH enzymes, namely Group VIIB and VIII, are intracellular (Table 2). The secreted Group VIIA phospholipase A2 features the catalytic Ser-His-Asp triad, the lipase motif (Fig. 3D), and shows broad substrate specificity including diacylglycerol and triacylglycerol hydrolase activity as well as phospholipase A1 activity. It hydrolyzes oxidized phospholipids associated with low-density lipoproteins and thus may inhibit the progression of atherosclerosis. However, other studies have suggested that Group VIIA phospholipase A2 is a risk factor for coronary artery disease. Thus, the role of Group VIIA phospholipase A2 in atherosclerosis and cardiovascular disease remains unclear. Group VIIB phospholipase A2 is an intracellular protein with an amino-terminal myristoylation site. Cell culture studies showed that Group VIIB phospholipase A2 translocated from the cytosol to membranes in the presence of oxidants, and that its overexpression protected cells from undergoing apoptosis upon exposure to oxidative stress. Thus, it has been suggested that Group VIIB phospholipase A2 may protect cells against oxidative stress. The other intracellular platelet activating factor acylhydrolase phospholipase A2 (Group VIII phospholipase A2) is a holoenzyme comprised of Group VIIIA and VIIIB subunits (Table 2) and a β regulatory subunit (encoded by the L1S gene). The Group VIIIA (α1) and Group VIIIB (α2) subunits are catalytic subunits and can form homo- or heterodimers. Depending upon the combination of subunits within the holoenzyme, the intracellular Group VIII phospholipase A2 isozymes assume different substrate specificities. Group VIIIB-null mice show reduced testis size, Group VIIIA-null mice appear normal, while double knockouts are sterile and show significant reduction of germ cells. These findings show that Group VIII phospholipase A2 is involved in spermatogenesis.
The lysosomal phospholipase A2 enzyme (Group XV phospholipase A2) (Table 2) is a Ca2+-independent enzyme with acyl-ceramide synthase, transacylase and lysophospholipase activities. Optimal activity of lysosomal phospholipase A2 occurs at pH 4.5 suggesting that the enzyme localizes to lysosomes. Lysosomal phospholipase A2 possesses the lipase motif (Gly-x-Ser-x-Gly), the catalytic Ser-Asp-His triad, a putative amino-terminal signal sequence and N-linked glycosylation sites (Fig. 3E). Lysosomal phospholipase A2-null mice showed an accumulation of phosphatidylcholine and phosphatidylethanolamine in alveolar macrophages suggesting that phospholipase A2-XV may be a major enzyme of pulmonary surfactant phospholipid degradation by alveolar macrophages. Disorders of surfactant metabolism present as respiratory deficiencies.
III. Phospholipase C
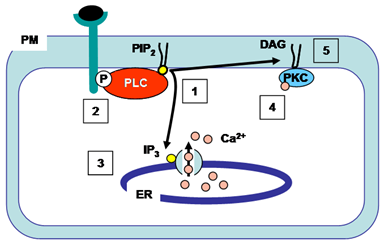
Phospholipase C enzymes cleave the glycerophosphate bond of phospholipids (Fig. 2). The phosphoinositide-specific phospholipase C family consists of 13 isozymes that are divided into six subfamilies – phospholipase C-β, -γ, -δ, -ε, -ζ, and –η, and each isozyme has more than one alternative splicing variant (Table 3). Activated phospholipase C enzymes catalyze the hydrolysis of phosphatidylinositol 4,5-bisphosphate within the membrane to generate the second messengers diacylglycerol and 1,4,5-triphosphate (Fig. 5). Diacylglycerol (which remains embedded in the membrane) and 1,4,5-triphosphate (which is released into the cytosol) initiate further signal transduction pathways through the activation of protein kinase C and intracellular Ca2+ release (Fig. 5). Studies have shown that phospholipase C-β are activated by G-protein-coupled receptors, phospholipase C-γ are activated by receptor tyrosine kinases, and phospholipase C-ε are activated by either pathway. The activation mechanisms for phospholipase C-δ, -η, and -ζ are not known.
|
Table 3. Phospholipase C family |
||||||
| Name | Splice variants | Tissue distribution | Activated by | |||
|---|---|---|---|---|---|---|
| PLC-β1 | β1a, β1b | Brain | G-protein coupled receptors | |||
| PLC-β2 | β2a, β2b | Brain & blood | ||||
| PLC-β3 | β3a, β3b | Brain, liver & ovary | ||||
| PLC-β4 | β4a, β4b | Brain & eye | ||||
| PLC-γ1 | γ1a, γ1b | Brain & embryo tissues | receptor-tyrosine kinases | |||
| PLC-γ2 | N/A | Lymph node | ||||
| PLC-δ1 | δ1a, δ1b | Brain & testis | ? | |||
| PLC-δ3 | δ3a, δ3b | Brain | ||||
| PLC-δ4 | δ4a, δ4b, δ4c | Brain | ||||
| PLC-ε | ε1a, ε1b, ε1c | Brain & connective tissue | G-protein coupled receptors or receptor-tyrosine kinases |
|||
| PLC-ζ | ζ1a, ζ1b, ζ1c | Testis | ? | |||
| PLC-η1 | η1a, η1b, η1c, η1d | Brain & testis | ? | |||
| PLC-η2 | η2a, η2b, η2c | Brain, eye & pancreas | ||||
| PLC, phospholipase C | ||||||
Figure 5. Action of phospholipase C. Activated phospholipase C hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) producing 1,4,5-triphosphate (IP3, which is released into the cytosol) and diacylglycerol (DAG, which remains in the plasma membrane) (1). This phospholipase C-γ isozyme is activated by binding to a specific phosphorylated tyrosine on the cytoplasmic side of the receptor after ligand binding (2). 1,4,5-Triphosphate (IP3) diffuses to the ER where it opens 1,4,5-triphosphate (IP3)-gated Ca2+ channels (3). Ca2+ in the cytosol acts as a second messenger by activating other signaling molecules such as calmodulin and protein kinase C. The initial rise in cytosolic Ca2+ induced by 1,4,5-triphosphate alters protein kinase C (PKC) such that it translocates from the cytosol to the cytoplasmic face of the plasma membrane (4). Diacylglycerol (DAG) activates Ca2+-dependent serine/threonine protein kinase C (PKC) which then phosphorylates target proteins. Ca2+, calcium ions; DAG, diacylglycerol; ER, endoplasmic reticulum; IP3, 1,4,5-triphosphate; P, phosphorylated tyrosine; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PLC, phospholipase C; PM, plasma membrane.
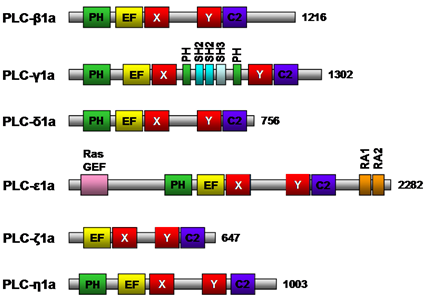
The members of the phospholipase C family share several conserved structural domains (Fig. 6). The pleckstrin homology domain mediates binding of phospholipase C to phospholipid; phospholipase C-δ binds to phosphatidylinositol 4,5-bisphosphate and phospholipase C-β2 and -β3 bind to phosphatidylinositol 3,4,5-bisphosphate. The X and Y structural domains are responsible for catalytic activity and are composed of alternating α-helices and β-strands, whereas the EF-hand motif is a helix-turn-helix structural domain that binds Ca2+ ions (Fig. 6). The Ca2+-dependent phospholipid binding domain (C2 domain) of phospholipase C-δ1 possesses three to four Ca2+ binding sites. The Src homology 2, Src homology 3, and split PH domain of phospholipase C-γ1a (Fig. 6) have all been implicated in various protein/protein interactions. Phospholipase C - null mice studies have shed some light on the functions of these phospholipase C isozymes. Phospholipase C-β1-null mice experienced epileptic seizures and sudden death suggesting phospholipase C-β1 is essential for the normal functioning of inhibitory neuronal circuitry, phospholipase C-γ1-null mice die by embryonic day 9 highlighting the widespread importance of this enzyme, and phospholipase C-ε plays a role in heart development. Thus, phospholipase C isozymes have been implicated in a variety of different physiological roles in various tissues, including cell growth, cell differentiation and gene expression pathways. The specific roles and signal transduction networks of some phospholipase C isozymes remain to be determined.
Figure 6. Schematic structure of mouse phospholipase C isozymes. Representative isoforms of each class of mouse phospholipase C with positions of the pleckstrin homology (PH) domain, EF-hand motif (EF), catalytic X and Y domain, and Ca2+-dependent phospholipid binding domain (C2) are shown. In phospholipase C-γ1a, two SH2, one SH3 and a split PH domain are indicated in aqua, light blue, and green, respectively. In phospholipase C-ε1a, RasGEF, RA1 & 2 are indicated by pink and orange, respectively. The number of amino acid residues for each isozyme is indicated to the right. C2, Ca2+-dependent phospholipid binding domain; EF, EF-hand motif; PH, pleckstrin homology domain; Ras GEF, Ras guanine nucleotide exchange factor; SH, Src homology.
IV. Phospholipase D
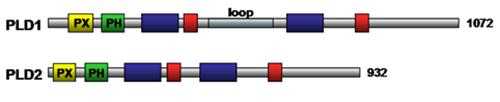
Phospholipase D activity (removal of phospholipid head group (Fig. 2)) has been detected in viruses, plants and animals. There are two isoforms of mammalian phospholipase D enzymes, namely, phospholipase D1 (120 kDa) and phospholipase D2 (105 kDa). These phospholipase D isozymes are regulated by a variety of molecules including protein kinases, polyphosphoinositides, and myristoylated alanine-rich C kinase substrate (MARKS) protein (see below). Phospholipase D and the product of its catalytic reaction phosphatidic acid have been implicated in regulating a diverse range of cellular processes including inflammation, control of intracellular membrane transport, neuronal and cardiac stimulation, cell migration, and chemo-resistance. Both phospholipase D1 and phospholipase D2 require a cofactor for activity. The basal activity of phospholipase D1 is low and can be stimulated by phosphatidylinositol 4,5-bisphosphate, phosphatidylinositol 3,4,5-bisphosphate, protein kinase Cα, and GTP-binding proteins (Rho, Rac Arf, CDC42). The activity of phospholipase D2 is positively regulated by phosphatidylinositol 4,5-bisphosphate and Ral, and negatively regulated by cytoskeletal proteins. The regulatory domains of phospholipase D enzymes include phox homology, and pleckstrin homology domains which are involved in lipid binding (Fig. 7). The catalytic core of eukaryotic phospholipase D enzymes consists of four conserved domains, of which domains I and III are the catalytic domains whereas II and IV contain the charged motif, HxK(x)4D(x)6GSxN, that plays a role in catalysis as well (Fig. 7). Mutational studies have suggested that the loop region of phospholipase D1 (Fig. 7) contains a regulatory element, such that deletion of the loop resulted in increased phospholipase D activity.
Figure 7. Schematic structure of mammalian phospholipase D enzymes. Positions of the PX domain, PH domain, phospholipase D-specific catalytic domains, HKD domain, and loop are indicated in yellow, green, dark blue, red and light blue, respectively. The number of amino acid residues for each enzyme is indicated to the right. PH, pleckstrin homology; PX, phox homology domain.
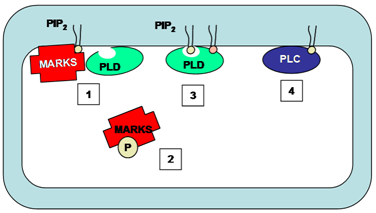
MARCKS and MARCKS-related protein regulate phosphatidylinositol 4,5-bisphosphate availability in a wide variety of cell types and thus regulate the activity of both phospholipase C and phospholipase D enzymes, among other functions. All functions of MARCKS and MARCKS-related proteins are related to the phosphatidylinositol 4,5-bisphosphate binding properties of these proteins. MARCKS co-localizes with phosphatidylinositol 4,5-bisphosphate in membrane microdomains. Association of MARCKS with phosphatidylinositol 4,5-bisphosphate inhibits phospholipase C-catalyzed hydrolysis of phosphatidylinositol 4,5-bisphosphate thus regulating phospholipase C activity (Fig. 8). Phosphorylation of MARCKS by protein kinase C results in translocation of the protein from membranes to cytosol, releasing the inhibition and allowing phosphatidylinositol 4,5-bisphosphate-mediated phospholipase D activity or phosphatidylinositol 4,5-bisphosphate cleavage via phospholipase C (Fig. 8).
Figure 8. The regulation of phospholipase C and phospholipase D by MARKS. MARKS co-localizes with PIP2 on the membrane. The binding of MARKS to PIP2 prevents this phospholipid from binding to and activating phospholipase D (1). Upon phosphorylation of the MARKS protein, it dissociates from the membrane into the cytosol (2). PIP2 is then available as a cofactor for phospholipase D activity (3) or as a substrate for phospholipase C-catalyzed cleavage (4). MARKS, myristoylated alanine-rich C kinase substrate; PLC, phospholipase C; PLD, phospholipase D.
V. Special Phospholipases – Lipase Gene Family
The secreted phospholipase A1 isozymes are part of the broad lipase gene family that extends from insects to mammals (see section II). The classical members of this gene family include lipoprotein lipase, hepatic lipase, endothelial lipase, pancreatic lipase and pancreatic lipase-related protein-2, which share a similar physiological role in the clearance of dietary triacylglycerol. Pancreatic lipase and pancreatic lipase-related protein-2 are synthesized in acinar cells, secreted into the intestinal tract, and play a role in the absorption of fatty acids by hydrolyzing triacylglycerol (Table 1). Lipoprotein lipase is synthesized mainly by adipocytes and muscle cells, and hydrolyzes triacylglycerol from circulating triacylglycerol-rich lipoproteins (Table 1). Hepatic lipase and endothelial lipase are synthesized by hepatocytes and endothelial cells, respectively, and hydrolyze triacylglycerol and phospholipids associated with triacylglycerol-rich lipoproteins as well as high-density lipoproteins in the plasma (Table 1).
The majority of lipoprotein lipase, hepatic lipase and endothelial lipase are anchored to the endothelium via heparan suflfate proteoglycans. In addition to their catalytic roles in hydrolyzing plasma lipids, hepatic lipase, lipoprotein lipase and endothelial lipase also possess noncatalytic roles in the clearance of lipids and lipoprotein particles through receptor-mediated endocytosis or a process termed selective lipid uptake. Furthermore, a recent study revealed that hepatic lipase may have an intracellular role in impairing the hepatic secretion of triacylglycerol-rich particles. The plasma levels and activity of lipoprotein lipase, hepatic lipase and endothelial lipase are modulated by several extracellular factors, including angiopoietin-like 3 protein.
VI. Other Phospholipases – Patatin
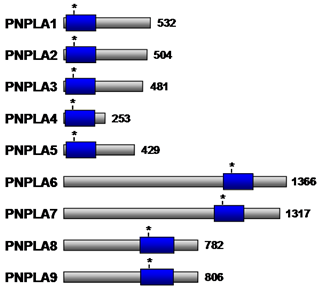
Structural analysis of a plant lipid acylhydrolase, patatin, has revealed the presence of a catalytic Ser-Asp dyad and an active site that is structurally similar to cytosolic phospholipase A2α. Importantly, however, patatin does not exhibit interfacial activation, presumably owing to the absence of a flexible lid covering the active site. Patatin-like phospholipase domain containing enzymes (PNPLA) have also been found in animals. PNPLA also contain the conserved serine lipase motif (Gly-x-Ser-x-Gly) (Fig. 9), and exhibits nonspecific acylhydrolase activity. Apart from the catalytic Ser-Asp dyad, PNPLA are characterized by three layer α/β/α architecture that differs from the basic lipase α/β hydrolase folds of cytosolic phospholipase A2α. Nine PNPLA family members (PNPLA1-9) (Table 4) are present in various tissues in humans and play a role in a variety of cellular processes. Although the physiological functions of some members remain to be elucidated, PNPLA2 and PNPLA3, which are highly expressed in adipose tissue, appear to play a role in regulating concentrations of stored lipids under different nutritional conditions. PNPLA6 is associated with the endoplasmic reticulum and Golgi apparatus in neurons and has been suggested to play a role in maintaining axon integrity, while PNPLA8 is a myocardial phospholipase that maintains mitochondrial integrity.
Figure 9. Schematic representation of the PNPLA family members. The patatin phospholipase domain is shown as a blue box. The asterisk denotes the Ser residue within the predicted active site. The number of amino acid residues for each enzyme is indicated to the right. PNPLA, patatin-like phospholipase domain containing enzymes.
|
Table 4. Patatin-like phospholipase domain containing family members |
||||||
| Name | Enzymatic function | Tissue and cellular expression | Physiological function | |||
|---|---|---|---|---|---|---|
| PNPLA1 | unknown | ? | ? | |||
| PNPLA2 | triacylglycerol hydrolase; transacylase; PLA2 |
white and brown adipose tissue; cytosolic lipid droplets | key enzyme in hydrolysis of stored triacylglycerol | |||
| PNPLA3 | triacylglycerol hydrolase, transacylase; PLA2 |
white adipose tissue; cellular membranes |
responds to nutritional status; biochemical function unclear | |||
| PNPLA4 | retinoic acid hydrolase; acylglycerol & retinol transacylase; triacylglycerol hydrolase; PLA2 |
keratinocytes | physiological relevance unclear | |||
| PNPLA5 | low levels | responds to nutritional status; function inconclusive | ||||
| PNPLA6 | in vitro: esterase, hydrolase | nervous system; endoplasmic reticulum & Golgi apparatus | critical function in maintaining axonal integrity across species | |||
| PNPLA7 | lysophospholipid hydrolase | prostate, pancreas, white adipose tissue; endoplasmic reticulum and small lipid droplets | responds to nutritional status; energy metabolism; further clarification required | |||
| PNPLA8 | PLA1 and PLA2 | heart; mitochondria and peroxisomes | important in cardiac phospholipid homeostasis and mitochondrial function | |||
| PNPLA9 | PLA2; acetyl hydrolase (against platelet activating factor) | ubiquitously expressed; cytoplasm and plasma membranes | phospholipid remodeling; signal transduction; cell proliferation; apoptotic death | |||
| PLA, phospholipase; PNPLA, patatin-like phospholipase domain containing enzymes | ||||||
In summary, phospholipases represent a complex cellular mechanism in regulating turnover and remodeling of phospholipids, which has a broad implication in a wide range of intracellular and extracellular functions. The products of phospholipase reactions are an integral component of a large number of signaling and regulatory pathways. Our understanding of the large array of phospholipase family members and their regulation has enhanced tremendously over the past decades. As a consequence, some phospholipases have been identified as therapeutic targets for prevention and treatment of diseases. What waits ahead is the full understanding of cellular and molecular mechanisms by which this large family of phospholipases exerts their impact under different physiological conditions.
Acknowledgment. The authors thank Dr. Maroun Bou Khalil for a critical reading of the manuscript.
In summary, phospholipases represent a complex cellular mechanism in regulating turnover and remodeling of phospholipids, which has a broad implication in a wide range of intracellular and extracellular functions. The products of phospholipase reactions are an integral component of a large number of signaling and regulatory pathways. Our understanding of the large array of phospholipase family members and their regulation has enhanced tremendously over the past decades. As a consequence, some phospholipases have been identified as therapeutic targets for prevention and treatment of diseases. What waits ahead is the full understanding of cellular and molecular mechanisms by which this large family of phospholipases exerts their impact under different physiological conditions.
Acknowledgment. The authors thank Dr. Maroun Bou Khalil for a critical reading of the manuscript.
Reading List
- Bou Khalil, M., Hou, W., Zhou, H., Elisma, F., Swayne, L.A., Blanchard, A.P., Yao, Z., Bennett, S.A. and Figeys, D. Lipidomics era: accomplishments and challenges. Mass Spectrom. Rev., 29, 877-929 (2010) (DOI: 10.1002/mas.20294).
- Jaye, M., Lynch, K.J., Krawiec, J., Marchadier, D., Maugeais, C., Doan, K., South, V., Amin, D., Perrone, M. and Rader, D.J. A novel endothelial-derived lipase that modulates HDL metabolism. Nat. Genet., 21, 424-428 (1999) (DOI: 10.1038/7766).
- Aoki, J., Inoue, A., Makide, K., Saiki, N. and Arai, H. Structure and function of extracellular phospholipase A1 belonging to the pancreatic lipase gene family. Biochimie, 89,, 197-204 (2007) (DOI: 10.1016/j.biochi.2006.09.021).
- Schaloske, R.H. and Dennis, E.A. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta, 1761, 1246-1259 (2006) (DOI: 10.1016/j.bbalip.2006.07.011).
- Burke, J.E. and Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res., 50 Suppl., S237-S242 (2009) (DOI: 10.1194/jlr.R800033-JLR200).
- McKew, J.C., Lee, K.L., Shen, M.W., Thakker, P., Foley, M.A., Behnke, M.L., Hu, B., Sum, F.W., Tam, S., Hu, Y., Chen, L., Kirincich, S.J., Michalak, R., Thomason, J., Ipek, M., Wu, K., Wooder, L., Ramarao, M.K., Murphy, E.A., Goodwin, D.G., Albert, L., Xu, X., Donahue, F., Ku, M.S., Keith, J., Nickerson-Nutter, C.L., Abraham, W.M., Williams, C., Hegen, M. and Clark, J.D. Indole cytosolic phospholipase A2α inhibitors: discovery and in vitro and in vivo characterization of 4-ethyl)-1-(diphenylmethyl )-1H-indol-3-yl]propyl}benzoic acid,4-{3-[5-chloro-2-(2-{[3,4-dichlorobenzyl)sulfonyl]amino}ethyl)-1-(diphenylmethyl)-1H-indol-3-yl]propyl}benzoic acid, efipladib. J. Med. Chem., 51, 3388-3413 (2008) (DOI: 10.1021/jm701467e).
- Yaksh,T.L., Kokotos, G., Svensson, C.I., Stephens, D., Kokotos, C.G., Fitzsimmons, B., Hadjipavlou-Litina, D., Hua, X.Y. and Dennis, E.A. Systemic and intrathecal effects of a novel series of phospholipase A2 inhibitors on hyperalgesia and spinal prostaglandin E2 release. J. Pharmacol. Exp. Ther., 316, 466-475 (2006) (DOI: 10.1124/jpet.105.091686).
- Balsinde, J. and Balboa, M.A. Cellular regulation and proposed biological functions of group VIA calcium-independent phospholipase A2 in activated cells. Cell Signal., 17, 1052-1062 (2005) (DOI: 10.1016/j.cellsig.2005.03.002).
- Atsumi, G., Murakami, M., Kojima, K., Hadano, A., Tajima, M. and Kudo, I. Distinct roles of two intracellular phospholipase A2s in fatty acid release in the cell death pathway. Proteolytic fragment of type IVA cytosolic phospholipase A2α inhibits stimulus-induced arachidonate release, whereas that of type VI Ca2+-independent phospholipase A2 augments spontaneous fatty acid release. J. Biol. Chem., 275, 18248-18258 (2000) (DOI: 10.1074/jbc.M000271200).
- Hiraoka, M., Abe, A. and Shayman, J.A. Structure and function of lysosomal phospholipase A2: identification of the catalytic triad and the role of cysteine residues. J. Lipid Res., 46, 2441-2447 (2005) (DOI: 10.1194/jlr.M500248-JLR200).
- Suh, P.G., Park, J.I., Manzoli, L., Cocco, L., Peak, J.C., Katan, M., Fukami, K., Kataoka, T., Yun, S. and Ryu, S.H. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB. Rep., 41, 415-434 (2008).
- Golebiewska, U. and Scarlata, S. The effect of membrane domains on the G protein-phospholipase Cβ signaling pathway. Crit. Rev. Biochem. Mol. Biol., 45, 97-105 (2010) (DOI: 10.3109/10409231003598812).
- McDermott, M., Wakelam, M.J. and Morris. A.J. Phospholipase D. Biochem. Cell Biol., 82, 225-253 (2004) (DOI: 10.1139/o03-079).
- Rudge, S.A. and Wakelam, M.J. Inter-regulatory dynamics of phospholipase D and the actin cytoskeleton. Biochim. Biophys. Acta, 1791, 856-861 (2009) (DOI: 10.1016/j.bbalip.2009.04.008).
- Sundaram, M., Cook, H.W. and Byers, D.M. The MARCKS family of phospholipid binding proteins: regulation of phospholipase D and other cellular components. Biochem. Cell Biol., 82, 191-200 (2004) (DOI: 10.1139/o03-087).
- McCoy, M.G., Sun, G.S., Marchadier, D., Maugeais, C., Glick, J.M. and Rader, D.J. Characterization of the lipolytic activity of endothelial lipase. J. Lipid Res., 43, 921-929 (2002).
- Bamji-Mirza, M., Sundaram, M., Zhong, S., Yao, E.F., Parks, R.J. and Yao, Z. Secretion of triacylglycerol-poor VLDL particles from McA-RH7777 cells expressing human hepatic lipase. J. Lipid Res., 52, 540-548 (2011) (DOI: 10.1194/jlr.M012476).
- Andrews, D.L., Beames, B., Summers, M.D. and Park, W.D. Characterization of the lipid acyl hydrolase activity of the major potato (Solanum tuberosum) tuber protein, patatin, by cloning and abundant expression in a baculovirus vector. Biochem. J., 252, 199-206 (1988).
- Kienesberger, P.C., Oberer, M., Lass, A. and Zechner, R. Mammalian patatin domain containing proteins: a family with diverse lipolytic activities involved in multiple biological functions. J. Lipid Res., 50 Suppl., S63-S68 (2009) (DOI: 10.1194/jlr.R800082-JLR200).
In This Section
- Glycerophosphate and Acylglycerophosphate Acyltransferases
- Mammalian Diacylglycerol Acyltransferases (DGAT)
- Fatty Acid beta-Oxidation
- Regulation of Lipins and Their Role in Lipid Metabolism
- Phospholipid Biosynthesis
- Phospholipases
- Acylglycerol Lipases (Neutral Lipid Hydrolysis)
- Metabolism and Function of Very-Long-Chain Polyunsaturated Fatty Acids (>C24) in Mammals