Glycerophosphate and Acylglycerophosphate Acyltransferases
The Author: Hei Sook Sul, Doris H. Calloway Chair in Human Nutrition, Department of Nutritional Science and Toxicology, University of California, Berkeley, 94720, U.S.A. DOI: 10.21748/lipidlibrary.39185
1. Pathway of de novo Phosphatidic Acid Biosynthesis
1,2-Diacyl-sn-glycerol-3-phosphate (phosphatidic acid) is a key intermediate in the synthesis of glycerophospholipids and triacylglycerols. Phospholipids are the predominant component of biomembranes and determine such properties as membrane permeability and the activity of membrane proteins. Triacylglycerols are the major storage form of energy in mammals and are also components of lipoproteins and milk. Furthermore, 1-acylglycerol-3-phosphate (lysophosphatidic acid) and phosphatidic acid can serve as signaling molecules.
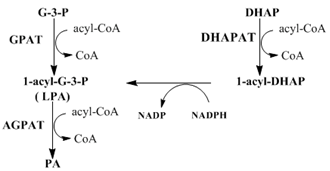
Half a century ago, Kennedy and coworkers first described the pathways of phosphatidic acid biosynthesis as well as its conversion to phospholipids and triacylglycerols. In de novo biosynthesis of phosphatidic acid, glycerol-3-phosphate is esterified with a fatty acyl-CoA at the sn-1 position catalyzed by glycerol-3-phosphate acyltransferase (GPAT) to form lysophosphatidic acid [1,2]. Lysophosphatidic acid is esterified at the sn-2 position with a fatty acyl-CoA catalyzed by 1-acylglycerol-3-phosphate acyltransferase (AGPAT, also called lysophosphatidic acid acyltransferase (LPAAT)) to form phosphatidic acid. Phosphatidic acid can then be used for the synthesis of phospholipids and triacylglycerols. In addition to this glycerol-3-phosphate pathway, the dihydroxyacetone phosphate (DHAP) pathway is also used in the synthesis of phosphatidic acid. DHAP can be first esterified to form 1-acyldihydroxyacetone-phosphate catalyzed by dihydroxyacetone-phosphate acyltransferase (DHAPAT), and 1-acyldihydroxyacetone-phosphate is the precursor for the ether lipid biosynthesis. However, 1-acyldihydroxyacetone-phosphate can also be reduced to lysophosphatidic acid catalyzed by 1-acyl/1-akyl dihydroxyacetone-phosphate reductase (ADHAPR) before further esterification to form phosphatidic acid.
Most naturally occurring glycerophospholipids have an asymmetric distribution of saturated and unsaturated fatty acids, with saturated fatty acids preferentially esterified in the sn-1 position and unsaturated fatty acids in the sn-2 position. Some tissues such as testis, retina, and brain contain a high level of glycerophospholipids with polyenoic acids of chain lengths greater than 22 carbons often present at the sn-2 position. It is believed that the fatty acyl-CoA substrate preferences of acyltransferases in de novo phosphatidic acid synthesis as well as those for deacylation and reacylation of phospholipids during remodeling (Lands cycle) may contribute to this asymmetric distribution. In this section, we will discuss the state of our understanding of the mechanisms and regulation of the mammalian acyltransferases involved in de novo synthesis of phosphatidic acid (Figure 1). The monoacylglycerol pathway that occurs in the intestine during fat absorption will not be discussed.
Figure 1. Phosphatidic acid biosynthetic pathways
2. Mammalian Acyltransferases in Phosphatidic Acid Biosynthesis
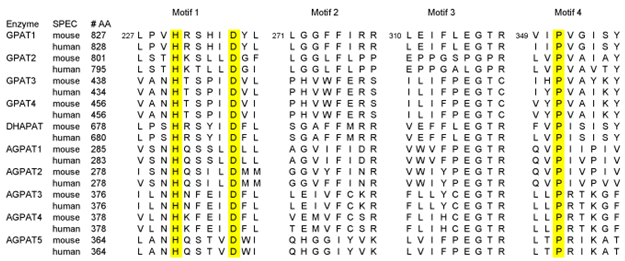
GPAT activity has been known to be present in both mitochondria and in the endoplasmic reticulum (ER). GPAT activity in mitochondria was regarded to be resistant to the sulfhydryl group-modifying reagent, N-ethylmaleimide, whereas GPAT activity in the ER was sensitive to N-ethylmaleimide and accounted for the majority of total GPAT activity in most tissues. In 1993, the N-ethylmaleimide-resistant mitochondrial GPAT1 was cloned and this was the first cloning of a mammalian enzyme in the glycerophospholipid biosynthetic pathway [3,4]. GPAT1 contains the acyltransferase domain (PF01553) of approximately 150 amino acids in length, subsequently known to be present in various acyltransferases including GPAT, AGPAT, DHAPAT, and lysophospholipid acyltransferase (LPLAT), as well as several proteins whose activities are yet to be understood (Table 1).
Table 1. Sequence comparison of conserved motifs in acyltransferase domain of murine and human GPATs, DHAPAT and AGPATs
Considerable GPAT activity in mitochondria from GPAT1 knockout mice indicated the presence of a second mitochondrial GPAT. The availability of mouse and human genome sequence data led to identification of the second mitochondrial GPAT, GPAT2, having 32% identity and 72% similarity to GPAT1 in 2007 [2]. Likewise, the isoforms of ER-associated GPATs that are structurally close to each other, GPATs 3 and 4, were also identified [2,5]. DHAPAT is found in peroxisomes where ether lipid biosynthesis occurs [6]. A total of 11 proteins containing the conserved acyltransferase domain have been annotated as AGPATs. AGPAT6 exhibits GPAT activity and is now known as GPAT4 [7]. AGPAT10 has not only AGPAT but also GPAT activity and is now named GPAT3 [5,7]. In addition, several members (AGPATs [7-9,11]) of this family exhibit other enzyme activities, including LPLAT activities with acceptor preference for differing lysophospholipids and thus are not discussed in this section. At least 5 isoforms of AGPATs (AGPATs 1-5) have been identified so far, although AGPAT activity of AGPAT4 is yet to be demonstrated. Some of these AGPATs, AGPAT1 and AGPAT2 specifically, were able to complement the AGPAT encoding plsC mutation in E. coli strain JC201 [8,9]. Most of the isoforms of AGPATs examined are found in the ER, while AGPATs 3 and 5 are also found in Golgi and mitochondria, respectively [10,11].
3. Common Structural Characteristics of Acyltransferases
Due to their membrane association, mammalian GPATs and AGPATs are difficult to purify and reconstitute to active enzymes, and X-ray crystallography data are not available. Therefore substrate recognition and reaction mechanisms are not fully understood. Regardless, it can be predicted that the region(s) conserved between the GPAT and AGPAT are likely to be involved in the function shared between the two enzymes, such as fatty acyl-CoA binding or the esterification reaction. The acyltransferase domain found in GPAT and AGPAT contains four conserved motifs. The role of the conserved residues has been elucidated mainly by mutational analysis of E. coli GPAT that can use either acyl-CoA or acyl-ACP as a substrate as well as murine mitochondrial GPAT1 and human AGPAT1 [12-14]. The crystal structure of soluble squash plastid GPAT that uses acyl-ACP, but not acyl-CoA, and lacks Motifs 3 and 4, has provided additional information [15].
Motif 1 contains a conserved HX4D, and mutational studies of E. coli GPAT demonstrated the critical role of this conserved sequence for catalysis. Thus, mutations of histidine or aspartic acid residues of Motif 1 in GPAT appear to reduce catalytic activity without significant changes in apparent Km for either glycerol-3-phosphate or palmitoyl-CoA [1]. The authors of this study proposed that, by analogy to chloramphenicol acetyltransferase, histidine might function as a general base to deprotonate the hydroxyl moiety of the acyl acceptor and that aspartic acid may participate with histidine in a charge-relay system similar to that seen in serine proteases. In fact, mutations of corresponding histidine or aspartic acid residues of AGPAT1 cause a drastic decrease in acyltransferase activity [14]. Crystal structure of plastidial GPAT also supports the key catalytic role of the HX4D motif [15].
Because of the positively charged guanidino group, arginine is well suited to bind the phosphate group of glycerol-3-phosphate or acyl-CoA. Arginine-modifying agents, phenylglyoxal and cyclohexanedione, was reported to inactivate E. coli GPAT [1]. Since CoA protected GPAT from inactivation, they proposed that one or more arginine residues are at or near the active site of the enzyme. In this regard, both Motif 2 and Motif 3 contain conserved arginine residues and have been reported to be critical for binding of glycerol-3-phosphate and Km for this substrate was shown to increase upon mutations of these residues in E. coli GPAT [12]. Similarly, arginine-modifying agents also inhibited murine mitochondrial GPAT1 activity. However, CoA did not protect GPAT1 from inactivation [13]. Amino acid substitutions were made for R318 in GPAT1, as well as nearby F313 and E315 within the conserved seven amino acid stretch in Motif 3, in addition to the only remaining conserved arginine, R278 in Motif 2 of GPAT1. Single substitutions of R278, F313, or E315 with alanine decreased enzyme activity by 70–90%, demonstrating the importance of each of these residues for GPAT activity. More conservative substitutions of R278K, F313Y, and E315Q had a lesser or no effect on enzyme activity. In contrast, even a conservative substitution of R318K decreased enzyme activity by 90%, demonstrating that R318 is the most critical residue for GPAT1 activity. Moreover, the R278K mutant GPAT1 was still inactivated by arginine modifying agents, suggesting that R318 is probably responsible for the inactivation of GPAT1 by arginine-modifying agents. In this regard, although sensitivity of other GPAT isoforms to the arginine modifying agents is not known, GPAT2 also contains the arginine residue corresponding to this R318 of GPAT1, whereas GPAT3, and GPAT4 do not.
In mutational analysis of Motif 3, a dramatic decrease in Vmax in R318A or R318K mutant of GPAT1 was noted, whereas apparent Km for glycerol-3-phosphate or palmitoyl-CoA was not significantly affected [13]. Nor did the mutation of E315 of GPAT1 to glutamine affect the Km for glycerol-3-phosphate. These observations on murine GPAT1 suggest that Motif 3 may be involved in catalysis rather than binding of phosphate group of glycerol-3-phosphate or fatty acyl-CoA. In contrast, mutations of the corresponding conserved arginine residues at Motif 2 or Motif 3 of human AGPAT1 reduced affinity for lysophosphatidic acid but not for acyl-CoA, suggesting that these residues are critical for binding of phosphate group of lysophosphatidic acid [14]. Interestingly, plant GPATs do not contain conserved Motif 3.
Motif 4 has a conserved proline residue in all members of acyltransferase domain containing proteins. However, the signficance of the proline residue is not clear. Substitution mutations of this proline residue in AGPAT caused only a modest change in AGPAT activity. However, it is expected that the four conserved motifs in acyltransferases should act in concert in the acylation reaction. Further structural studies will be needed to understand the functional significance of motifs common in mammalian acyltransferases.
Acyltransferases are membrane proteins and the topology of some acyltransferases, such as GPAT1, AGPAT1 and AGPAT3, have been examined. GPAT1 resides at the mitochondria, with two transmembrane domains spanning the outer mitochondrial membrane. The N-terminal region containing all 4 conserved motifs and the C-terminus face the cytoplasm and the loop between these two regions faces the mitochondrial intermembrane space [16]. However, the reverse orientation of N- and C-termini of GPAT1 facing the mitochondrial intermembrane space has also been reported [17]. For AGPAT1, several transmembrane domains have been predicted, placing Motif 1 on the cytosolic side of the ER and Motif 3 on the lumenal side, with a transmembrane domain separating the motifs to opposite sides of the membrane [14]. Similarly, Motif 1 of AGPAT3 has been located to the cytoplasmic side and Motifs 2-4 at the lumenal side of the ER and Golgi [18]. One possibility is that, since all 4 motifs are critical for the acyltransferase activity, all motifs, either at the cytosolic or lumenal side, may be near the membrane. Further topological studies on various acyltransferases may help to reveal the mechanism of these enzymes.
4. GPAT Isoforms: Property and Regulation
Glycerol-3-phosphate acyltransferase (GPAT, EC 2.3.1.15) is the first committed, and presumed to be a rate-limiting step, in glycerophospholipid biosynthesis. It catalyzes esterification of glycerol-3-phosphate in the sn-1 position with a fatty acyl-CoA to form lysophosphatidic acid. As described above, so far four GPAT isoforms encoded by separate genes have been found in mammals. GPAT activity is present in mitochondria and in ER. In most tissues, mitochondrial GPAT activity comprises 10% of total GPAT activity, whereas it can represent 50% of total GPAT activity in liver.
GPAT1 resides in the outer mitochondrial membrane and shows preference for saturated long-chain acyl-CoA, particularly palmitoyl-CoA [13]. The unsaturated fatty acyl-CoAs, oleoyl-, linoleoyl-, linolenoyl- and arachidonoyl-CoA, are only about 20% effective as palmitoyl-CoA as a substrate. As mentioned above, GPAT1 differs from other isoforms in that sulfhydryl group modifying agents cannot inactivate its activity. GPAT2 also is a mitochondrial outer membrane protein but is inactivated by N-ethylmaleimide and does not have substrate preference for palmitoyl-CoA over oleoyl-CoA [2]. GPATs 3 and 4 reside in the ER membrane. GPATs 3 and 4 are sensitive to N-ethylmaleimide and do not have preference for the saturated fatty acyl-CoA as a substrate, but prefer fatty acyl-CoAs with 16 and 18 carbons in length [2,5]. GPAT3 and GPAT4 were initially classified as AGPATs and GPAT3 also shows AGPAT activity, but GPAT4 does not appear to contain AGPAT activity.
GPAT1 is highly expressed in liver and is the most critical isoform in triacylglycerol biosynthesis in this tissue. Thus, ablation of the GPAT1 gene or adenoviral knockdown of GPAT1 in mice causes lower liver triglyceride levels, whereas overexpression in liver causes an increase in hepatic and serum triglycerides. Mitochondrial GPAT has been known to have lower Km for its substrates, glycerol-3-phosphate and fatty acyl-CoA. Due to its low Km for fatty acyl-CoA, GPAT1 may direct fatty acyl-CoA for esterification by competing with carnitine-palmitoyl transferase-1 that directs fatty acyl-CoA for oxidation. Indeed, the rate of hepatic fatty acid oxidation and serum ketone body levels were higher in GPAT1 knockout mice [2]. In this regard, similar to other lipogenic enzymes such as fatty acid synthase and acetyl-CoA carboxylase, GPAT1 is under hormonal and nutritional control at the transcriptional level. GPAT1 expression in liver is very low in fasting/cAMP treatment, but is drastically upregulated by feeding/insulin treatment [19]. Binding of USF and SREBP-1c near the proximal promoter region of GPAT1 confers feeding/insulin dependent transcriptional activation [20,21]. ChREBP may also be involved since it binds to the promoter region [22].
It is noteworthy that only GPAT1 is under transcriptional regulation and GPATs 2-4 are largely unaffected. GPAT may also be regulated by a phosphorylation/dephosphorylation mechanism, and specific serine residues of GPAT1 that are phosphorylated have been detected [23]. Casein kinase 2 was reported to phosphorylate and activate mitochondrial GPAT, whereas AMP-activated protein kinase has been reported to inhibit mitochondrial GPAT activity [2]. However, specific site(s) of GPAT isoforms for phosphorylation by specific kinases have not been elucidated. Interestingly, GPAT2, the second mitochondrial GPAT, is most highly expressed in testis in comparison to other tissues including liver, and thus may have a yet to be defined unique role in testis.
Although GPAT1 expression increases during adipocyte differentiation, it comprises only 10% of total GPAT activity in adipose tissue. In contrast to liver, GPAT activity in ER accounts for the majority of GPAT activity in this tissue. Thus, GPAT3 is expressed at the highest level in visceral white adipose tissue and its expression increases drastically during adipocyte differentiation. GPAT3 is also found in other organs, including small intestine, kidney and heart, and its expression is relatively low in liver [5,7]. GPAT4 is highly expressed in brown adipose tissue, liver, mammary gland and testis, providing approximately 50% of total GPAT activity in liver and mammary gland. GPAT4 ablation in mice reduces liver triacylglycerol content and subcutaneous adipose tissue mass and these mice are resistant to diet-induced obesity [24]. Although GPAT4 is highly expressed in brown adipose tissue, defects in thermogenesis have not been observed in GPAT4 knockout mice. Triacylglycerol content of milk from GPAT4 knockout dams is extremely low and thus their pups die within a few days after birth [7]. GPAT4 is also found in spermatocytes and spermatids during meiosis and thus may play a role in spermatogenesis.
5. DHAP Acyltransferase in Lysophosphatidic Acid Biosynthesis
As shown in Figure 1, an additional route of generating phosphatidic acid is to esterify dihydroxyacetone-phosphate catalyzed by dihydroxyacetone-phosphate acyltransferase (DHAPAT, EC 2.3.1.42) in the sn-1 position with fatty acyl-CoA to form 1-acyl dihydroxyacetone-phosphate (DHAP). 1-Acyl DHAP is converted into 1-alkyl DHAP catalyzed by alkyl-DHAP synthase for biosynthesis of ether lipids, alkyl glycerol ethers and plasmalogens. However, 1-acyl DHAP can be reduced to lysophosphatidic acid catalyzed by 1-acyl/1-alkyl dihydroxyacetone-phosphate reductase using NADPH (ADHAPR), before acylation of the sn-2 position by AGPAT to form phosphatidic acid.
DHAPAT contains the conserved acyltransferase domain as well as a typical peroxisome targeting signal type 1 (alanine-lysine-leucine) at the extreme C-terminus [6,25]. DHAPAT is highly expressed in murine heart, liver and testis and is found at lower levels in brain, lung and kidney. DHAPAT as well as ADHAPR is induced during adipocyte differentiation, while 1-alkyl DHAP synthase is decreased substantially during the same period. The degree of contribution of DHAP pathway for overall glycerolipid synthesis is not clear. It was reported that up to half of glycerolipid can be made through the DHAP pathway in 3T3-L1 adipocytes [26]. However, peroxisome disorder patients show deficiency in ether lipids, but not glycerolipids. Physiological conditions, including availability of substrates and NADPH/NADH ratio, may control the specific route(s), GPAT or DHAPAT, in generating lysophosphatidic acid in mammals.
6. AGPAT Isoforms: Property and Regulation
Lysophosphatidic acid is acylated in the sn-2 position by 1-acylglycerol-3-phosphate acyltransferase (AGPAT, EC 2.3.1.51) to form phosphatidic acid. At least 5 isoforms of AGPATs encoded by separate genes have been found. As mentioned above, however, a total of 11 proteins containing the conserved acyltransferase motifs are annotated as AGPATs. Two of them are now named as GPATs. Others exhibit different enzyme activities, such as lysophospholipid acyltransferase (LPLAT) activities and are not discussed further. Conversely, CGI-58/ABHD5 and endophilin-1 that do not contain the conserved acyltransferase domain have been reported to exhibit AGPAT activity.
AGPAT isoforms are mainly found in ER, while AGPAT3 and AGPAT5 are also detected in Golgi and mitochondria, respectively. These isoforms may have different substrate specificity. With differing intracellular localization, some of these AGPAT isoforms may have localized effects in generating signaling mediators or in membrane structure. However, specific functions of AGPAT isoforms are not well understood. AGPATs 1 and 2 are found in ER and are structurally related among the AGPAT family members. Whereas AGPAT1 is ubiquitously expressed and is especially high in skeletal muscle, AGPAT2 is highly expressed in liver, muscle and intestine, but also is the most highly expressed AGPAT in adipose tissue. AGPATs 1 and 2 show a subtle difference in their substrate preference: Myristoyl-, palmitoyl- and linoleoyl-CoA were shown to be most active in the AGPAT1 reaction in one report and arachidonoyl-CoA was well utilized in another report. In contrast, oleoyl-CoA is the most actively used in the AGPAT2 reaction [27].
AGPAT2 deficiency causes lipodystrophy in mice and humans, whereas AGPAT1 deficiency does not [28]. Lipodystrophy in AGPAT2 deficiency may not entirely be due to the inability to synthesize and store triacylglycerol in adipose tissue. Regardless, excess triacylglycerol that accumulates in liver and muscle suggests importance of AGPAT2 in triacylglycerol synthesis and storage in adipose tissue. It has been proposed that impaired adipocyte differentiation possibly due to impairment in phospholipid synthesis may also contribute to lipodystrophy syndrome in AGPAT2 deficiency [29]. However, AGPAT2 knockout mice do not show defects in early adipogenesis or proliferation but they manifest adipose tissue necrosis [30]. As with other lipogenic enzymes, AGPAT2 is overexpressed in certain cancers, and inhibition of AGPAT induces cell growth arrest and death [31,32].
AGPAT3 not only has AGPAT activity with a preference for lysophosphatidic acid with oleate at its sn-1 position and oleoyl-CoA as the acyl donor, but also has modest LPLAT activity that prefers arachidonoyl-CoA as the acyl donor [33], indicating a potential dual role of AGPAT3 in de novo glycerophospholipid synthesis and phospholipid remodeling. AGPAT3 is ubiquitously expressed but is detected at a high level in tissues such as testis and pancreas. Thus, AGPAT3 may provide a supply of polyunsaturated fatty acids including arachidonic acid in specific tissues, for example, testis [34]. AGPAT3 is found in ER and in the Golgi complex, and AGPAT3 has been proposed to affect Golgi structure and membrane trafficking by remodeling of phospholipids [10]. AGPAT4 is structurally related to AGPAT3 but its AGPAT activity is yet to be demonstrated. Similar to AGPAT3, AGPAT4 is also found in all tissues but present at a much lower level in some tissues including kidney, liver and muscle. The expression pattern of AGPAT5 is very similar to AGPAT4 and is highly expressed in testis. AGPAT5 not only exhibits AGPAT activity but also LPLAT activity. However, in contrast to AGPAT3, AGPAT5 prefers oleoyl-CoA for its LPLAT activity when lysophosphatidylethanolamine is used as the acceptor [11]. When overexpressed, AGPAT5 can be detected in ER as well as in mitochondria [11]. The catalytic activities indicate that AGPATs 3 and 5 appear to be at least two orders of magnitude less efficient than AGPATs 1 and 2. Interestingly, in comparison to AGPATs 1-2, AGPATs 3-5 are found at relatively higher levels in murine epidermis, where phospholipids can be essential for permeability barrier function [7]. Overall, AGPAT2 appears to be critical for triacylglycerol synthesis, whereas AGPATs 3-5 may serve for membrane phospholipid biosynthesis.
Not much is known about the regulation of AGPAT. AGPAT activity was reported to decrease in streptozotocin-diabetes, and insulin restored the activity. It has also been reported that AGPAT activity was increased rapidly in parotid gland lobules stimulated by isoproterenol or carbachol, and phosphatase treatment caused inactivation [35]. However, specific AGPAT isoform(s) responsive to hormonal regulation or their phosphorylation have not been studied. AGPAT3 mRNA levels in heart appear to increase when treated with a PPARα agonist, clofibrate [36]. Studies that address regulation of AGPAT at the gene expression level or by phosphorylation/dephosphorylation are needed.
7. Perspectives
Phospholipids are an integral component of all cellular membranes, such as ER, mitochondria, peroxisomes and plasma membrane. ER has been known to be the main intracellular organelle for glycerophospholipid biosynthesis. While two isoforms of GPAT are found in the ER, the other two are in the mitochondria. AGPATs are found mainly in the ER, but AGPAT3 and 5 can also be found in Golgi and mitochondria, respectively, and DHAPAT is found in peroxisomes. It is possible that lysophosphatidic acid produced by mitochondrial GPATs or by the peroxisomal DHAP pathway is transported to the ER for further esterification by AGPAT. However, the transport of lysophosphatidic acid between the intracellular organelles in phosphatidic acid synthesis has not been established. It is also possible that different isoforms may be used locally to meet the needs for membrane phospholipid and triacylglycerol synthesis or for other signaling and metabolic regulation. Thus, the specific function of various isoforms of acyltransferases at different intracellular organelles may be predicted but are not yet understood. Furthermore, potential regulation of specific isoforms at various levels, including gene expression and post-translational modification, has not been studied but is critical for understanding phospholipid and triacylglycerol biosynthesis.
The asymmetric distribution of fatty acids, with saturated fatty acids in the sn-1 position and unsaturated fatty acids in the sn-2 position is not well understood. Mitochondrial GPAT1 preferentially uses saturated over unsaturated fatty acyl-CoA as a substrate, which correlates with the presence of saturated fatty acids in the sn-1 position. Some of the AGPAT isoforms are shown to prefer oleoyl-CoA as a substrate. Although phospholipid remodeling may be critical for asymmetric distribution of fatty acids, better studies of enzyme kinetics and substrate preference as well as structure and topology of various isoforms of acyltransferases are needed. In addition, mass spectrometry-based lipidomics approaches of unbiased profiling coupled with overexpression or knockout techniques may provide information on the substrate specificity and function of these enzymes.
Acknowledgments
The author apologizes that, due to space limitations, many primary publications have not been cited. The work from the author’s laboratory was supported by grants from the NIDDK.
References
- Dircks, L. and Sul, H.S. Acyltransferases of de novo glycerophospholipid biosynthesis. Prog. Lipid Res., 38, 461-479 (1999) (DOI: 10.1016/S0163-7827(99)00012-0).
- Coleman, R.A. and Mashek, D.G. Mammalian triacylglycerol metabolism: synthesis, lipolysis, and signaling. Chem. Rev., 111, 6359-6386 (2011) (DOI: 10.1021/cr100404w).
- Yet, S.F., Lee, S., Hahm, Y.T. and Sul, H.S. Expression and identification of p90 as the murine mitochondrial glycerol-3-phosphate acyltransferase. Biochemistry, 32, 9486-9491 (1993) (DOI: 10.1021/bi00087a029).
- Yet, S.F., Moon, Y.K. and Sul, H.S. Purification and reconstitution of murine mitochondrial glycerol-3-phosphate acyltransferase. Functional expression in baculovirus-infected insect cells. Biochemistry, 34, 7303-7310 (1995) (DOI: 10.1021/bi00022a003).
- Gimeno, R.E. and Cao, J. Thematic review series: glycerolipids. Mammalian glycerol-3-phosphate acyltransferases: new genes for an old activity. J. Lipid Res., 49, 2079-2088 (2008) (DOI: 10.1194/jlr.R800013-JLR200).
- Ofman, R., Hogenhout, E.M. and Wanders, R.J. Identification and characterization of the mouse cDNA encoding acyl-CoA:dihydroxyacetone phosphate acyltransferase. Biochim. Biophys. Acta, 1439, 89-94 (1999) (DOI: 10.1016/S1388-1981(99)00081-5).
- Takeuchi, K. and Reue, K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab., 296, E1195-E1209 (2009) (DOI: 10.1152/ajpendo.90958.2008).
- West, J., Tompkins, C.K., Balantac, N., Nudelman, E., Meengs, B., White, T., Bursten, S., Coleman, J., Kumar, A., Singer, J.W. and Leung, D.W. Cloning and expression of two human lysophosphatidic acid acyltransferase cDNAs that enhance cytokine-induced signaling responses in cells. DNA Cell. Biol., 16, 691-701 (1997).
- Eberhardt, C., Gray, P.W. and Tjoelker, L.W. Human lysophosphatidic acid acyltransferase. cDNA cloning, expression, and localization to chromosome 9q34.3. J. Biol. Chem., 272, 20299-20305 (1997) (DOI: 10.1074/jbc.272.32.20299).
- Schmidt, J.A. and Brown, W.J. Lysophosphatidic acid acyltransferase 3 regulates Golgi complex structure and function. J. Cell. Biol., 186, 211-218 (2009) (DOI: 10.1083/jcb.200904147).
- Prasad, S.S., Garg, A. and Agarwal, A.K. Enzymatic activities of the human AGPAT isoform 3 and isoform 5: localization of AGPAT5 to mitochondria. J. Lipid Res., 52, 451-462 (2011) (DOI: 10.1194/jlr.M007575).
- Lewin, T.M., Wang, P. and Coleman, R.A. Analysis of amino acid motifs diagnostic for the sn-glycerol-3-phosphate acyltransferase reaction. Biochemistry, 38, 5764-5771 (1999) (DOI: 10.1021/bi982805d).
- Dircks, L.K., Ke, J. and Sul, H.S. A conserved seven amino acid stretch important for murine mitochondrial glycerol-3-phosphate acyltransferase activity. Significance of arginine 318 in catalysis. J. Biol. Chem., 274, 34728-34734 (1999) (DOI: 10.1074/jbc.274.49.34728).
- Yamashita, A., Nakanishi, H., Suzuki, H., Kamata, R., Tanaka, K., Waku, K. and Sugiura, T. Topology of acyltransferase motifs and substrate specificity and accessibility in 1-acyl-sn-glycero-3-phosphate acyltransferase 1. Biochim. Biophys. Acta, 1771, 1202-1215 (2007) (DOI: 10.1016/j.bbalip.2007.07.002).
- Turnbull, A.P., Rafferty, J.B., Sedelnikova, S.E., Slabas, A.R., Schierer, T.P., Kroon, J.T., Nishida, I., Murata, N., Simon, J.W. and Rice, D.W. Crystallization and preliminary X-ray analysis of the glycerol-3-phosphate 1-acyltransferase from squash (Cucurbita moschata). Acta Crystallogr. D. Biol. Crystallogr., 57, 451-453 (2001) (DOI: 10.1107/S0907444901000257).
- Gonzalez-Baró, M.R., Granger, D.A. and Coleman, R.A. Mitochondrial glycerol phosphate acyltransferase contains two transmembrane domains with the active site in the N-terminal domain facing the cytosol. J. Biol. Chem., 276, 43182-43188 (2001) (DOI: 10.1074/jbc.M107885200).
- Balija, V.S., Chakraborty, T.R., Nikonov, A.V., Morimoto, T. and Haldar, D. Identification of two transmembrane regions and a cytosolic domain of rat mitochondrial glycerophosphate acyltransferase. J. Biol. Chem., 275, 31668-31673 (2000) (DOI: 10.1074/jbc.M002963200).
- Schmidt, J.A., Yvone, G.M. and Brown, W.J. Membrane topology of human AGPAT3 (LPAAT3). Biochem. Biophys. Res. Commun., 397, 661-667 (2010) (DOI: 10.1016/j.bbrc.2010.05.149).
- Shin, D.H., Paulauskis, J.D., Moustaid, N. and Sul, H.S. Transcriptional regulation of p90 with sequence homology to Escherichia coli glycerol-3-phosphate acyltransferase. J. Biol. Chem., 266, 23834-23839 (1991) (www.jbc.org/content/266/35/23834.long).
- Jerkins, A.A., Liu, W.R., Lee, S. and Sul, H.S. Characterization of the murine mitochondrial glycerol-3-phosphate acyltransferase promoter. J. Biol. Chem., 270, 1416-1421 (1995) (DOI: 10.1074/jbc.270.3.1416).
- Wong, R.H. and Sul, H.S. Insulin signaling in fatty acid and fat synthesis: a transcriptional perspective. Curr. Opin. Pharmacol., 10, 684-691 (2010) (DOI: 10.1016/j.coph.2010.08.004).
- Guha, P., Aneja, K.K., Shilpi, R.Y. and Haldar, D. Transcriptional regulation of mitochondrial glycerophosphate acyltransferase is mediated by distal promoter via ChREBP and SREBP-1. Arch. Biochem. Biophys., 490, 85-95 (2009) (DOI: 10.1016/j.abb.2009.07.027).
- Bronnikov, G.E., Aboulaich, N., Vener, A.V. and Stralfors, P. Acute effects of insulin on the activity of mitochondrial GPAT1 in primary adipocytes. Biochem. Biophys. Res. Commun., 367, 201-207 (2008) (DOI: 10.1016/j.bbrc.2007.12.127).
- Vergnes, L., Beigneux, A.P., Davis, R., Watkins, S.M., Young, S.G. and Reue, K. Agpat6 deficiency causes subdermal lipodystrophy and resistance to obesity. J. Lipid Res., 47, 745-754 (2006) (DOI: 10.1194/jlr.M500553-JLR200).
- Thai, T.P., Heid, H., Rackwitz, H.R., Hunziker, A., Gorgas, K. and Just, W.W. Ether lipid biosynthesis: isolation and molecular characterization of human dihydroxyacetonephosphate acyltransferase. FEBS Lett., 420, 205-211 (1997) (DOI: 10.1016/S0014-5793(97)01495-6).
- Hajra, A.K., Larkins, L.K., Das, A.K., Hemati, N., Erickson, R.L. and MacDougald, O.A. Induction of the peroxisomal glycerolipid-synthesizing enzymes during differentiation of 3T3-L1 adipocytes. Role in triacylglycerol synthesis. J. Biol. Chem., 275, 9441-9446 (2000) (DOI: 10.1074/jbc.275.13.9441).
- Hollenback, D., Bonham, L., Law, L., Rossnagle, E., Romero, L., Carew, H., Tompkins, C.K., Leung, D.W., Singer, J.W. and White, T. Substrate specificity of lysophosphatidic acid acyltransferase β - evidence from membrane and whole cell assays. J. Lipid Res., 47, 593-604 (2006) (DOI: 10.1194/jlr.M500435-JLR200).
- Cortes, V.A., Curtis, D.E., Sukumaran, S., Shao, X., Parameswara, V., Rashid, S., Smith, A.R., Ren, J., Esser, V., Hammer, R.E., Agarwal, A.K., Horton, J.D. and Garg, A. Molecular mechanisms of hepatic steatosis and insulin resistance in the AGPAT2-deficient mouse model of congenital generalized lipodystrophy. Cell Metab., 9, 165-176 (2009) (DOI: 10.1016/j.cmet.2009.01.002).
- Gale, S.E., Frolov, A., Han, X., Bickel, P.E., Cao, L., Bowcock, A., Schaffer, J.E. and Ory, D.S. A regulatory role for 1-acylglycerol-3-phosphate-O-acyltransferase 2 in adipocyte differentiation. J. Biol. Chem., 281, 11082-11089 (2006) (DOI: 10.1074/jbc.M509612200).
- Vogel, P., Read, R., Hansen, G., Wingert, J., Dacosta, C.M., Buhring, L.M. and Shadoan, M. Pathology of congenital generalized lipodystrophy in Agpat2-/- mice. Vet. Pathol., 48, 642-654 (2011) (DOI: 10.1177/0300985810383870).
- Bonham, L., Leung, D.W., White, T., Hollenback, D., Klein, P., Tulinsky, J., Coon, M. de Vries, P. and Singer, J.W. Lysophosphatidic acid acyltransferase-β: a novel target for induction of tumour cell apoptosis. Expert Opin. Ther. Targets, 7, 643-661 (2003) (DOI: 10.1517/14728222.7.5.643).
- Rastegar, F., Gao, J.L., Shenaq, D., Luo, Q., Shi, Q., Kim, S.H., Jiang, W., Wagner, E.R., Huang, E., Gao, Y., Shen, J., Yang, K., He, B.C., Chen, L., Zuo, G.W., Luo, J., Luo, X., Bi, Y., Liu, X., Li, M., Hu, N., Wang, L., Luther, G., Luu, H.H., Haydon, R.C. and He, T.C. Lysophosphatidic acid acyltransferase beta (LPAATβ) promotes the tumor growth of human osteosarcoma. PLoS One, 5, e14182 (2010) (DOI: 10.1371/journal.pone.0014182).
- Yuki, K., Shindou, H., Hishikawa, D. and Shimizu, T. Characterization of mouse lysophosphatidic acid acyltransferase 3: an enzyme with dual functions in the testis. J. Lipid Res., 50, 860-869 (2009) (DOI: 10.1194/jlr.M800468-JLR200).
- Koeberle, A., Shindou, H., Harayama, T. and Shimizu, T. Role of lysophosphatidic acid acyltransferase 3 for the supply of highly polyunsaturated fatty acids in TM4 Sertoli cells. FASEB J., 24, 4929-4938 (2010) (DOI: 10.1096/fj.10-162818).
- Soling, H.D., Fest, W., Schmidt, T., Esselmann, H. and Bachmann, V. Signal transmission in exocrine cells is associated with rapid activity changes of acyltransferases and diacylglycerol kinase due to reversible protein phosphorylation. J. Biol. Chem., 264, 10643-10648 (1989) (www.jbc.org/content/264/18/10643.long).
- Lu, B., Jiang, Y.J., Zhou, Y., Xu, F.Y., Hatch, G.M. and Choy, P.C. Cloning and characterization of murine 1-acyl-sn-glycerol 3-phosphate acyltransferases and their regulation by PPARα in murine heart. Biochem. J., 385, 469-477 (2005) (DOI: 10.1042/BJ20041348).
In This Section
- Glycerophosphate and Acylglycerophosphate Acyltransferases
- Mammalian Diacylglycerol Acyltransferases (DGAT)
- Fatty Acid beta-Oxidation
- Regulation of Lipins and Their Role in Lipid Metabolism
- Phospholipid Biosynthesis
- Phospholipases
- Acylglycerol Lipases (Neutral Lipid Hydrolysis)
- Metabolism and Function of Very-Long-Chain Polyunsaturated Fatty Acids (>C24) in Mammals