Silver Ion Chromatography and Lipids, Part 4
The Author: Boryana Nikolova-Damyanova, Institute of Organic Chemistry, Centre of Phytochemistry, Sofia 1113, Bulgaria
- A. Introduction
- B. Principles of Silver Ion Complexation with Double Bonds
- C. Silver Ion Thin-Layer Chromatography
- D. Silver Ion High-Performance Liquid Chromatography
- E. Low-Pressure Silver Ion Column Chromatography
- F. Combined Chromatographic Techniques
- G. References
E. Low Pressure Silver Ion Column Chromatography
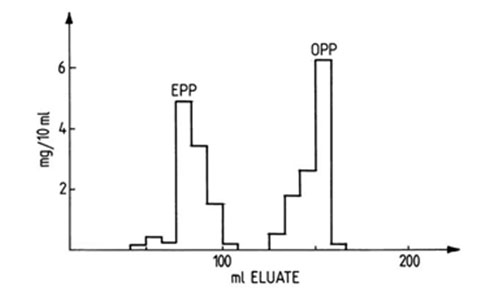
Column chromatography performed on silver ion-impregnated silica has as long a history as silver ion TLC, both techniques having been developed simultaneously and communicated in Chemistry and Industry (London) in the same year [1962]. De Vries [194,195] reported the first separations of fatty acid methyl esters and triacylglycerols and proposed a technique for preparation of the column that has been followed to some extent in all subsequent applications of the method [121]. Silicic acid (100 g) and aqueous silver nitrate (200 mL, 50% w/v) were mixed, and the slurry was heated for 30 minutes at 100°C, when it was cooled, filtered and dried for 16 hours at 120°C. The activated material was mixed with petroleum ether and used to pack the column; there was estimated to be 0.3 to 0.4 g silver nitrate per g silica [195]. By using a 11 cm high column and petroleum ether-benzene mixtures as the mobile phase, de Vries was able to separate fatty acids with zero to three double bonds, including cis- and trans-isomers (methyl elaidate and oleate) and triacylglycerols with zero to three double bonds, including EPP and OPP (Fig. 19). Sample loads varied between limits of 20 to 120 mg, while the purity of the fractions was estimated to be about 95%. Later, others extended the technique to the separation of fatty acid methyl esters with up to 6 double bonds by using stepwise elution with petroleum ether and increasing amounts (2 to 90%) of diethyl ether [94]. Recently, a small-scale separation of different plant oils, animal fats and plasma triacylglycerols on a mini-column (Pasteur pipette) by analogous means was described [106]. The triacylglycerols were separated into four fractions, i.e. with zero plus one, two, three and four or more double bonds by using hexane-diethyl ether mixtures as eluents.
Figure 19. Separation of elaidodipalmitin (EPP) and oleodipalmitin (OPP) by normal pressure column chromatography on silica gel G impregnated with silver nitrate [194]. Triacylglycerols were eluted with benzene-petroleum ether in steps from 40:60 to 100:0 (v/v). (Reproduced by kind permission of the author and of Chemistry and Industry (London), and redrawn from the original.)
As an alternative, macroreticular sulfonic acid ion-exchange resins were utilized as sorbents for silver ion column chromatography [4-9,59,66-68,171,172,174,206]. The technique has much in common with HPLC, since part of instrumentation is the same (pumps and detectors, for example). The main reason for discussing it in this section rather than the next is because of the much larger particle size (20-270 mesh), and because the pressure applied to the column is generally lower. There is, however, no distinct boundary between the two techniques. In the first experiments, Amberlyst XN 1005™ (not available commercially) was utilized [67], and it was loaded with silver ions by passing an aqueous silver nitrate solution through a column of resin until excess silver ions started to elute. The column was then washed with water and methanol, while methanol was used further as the mobile phase. Relatively pure fractions of saturated, trans- and cis-monoenoic fatty acid methyl esters were obtained with the first column [67], and some fractionation of positional isomers in the monoenoic fraction was even observed. Further attempts were made to improve the separation and to resolve positional [172] and geometrical isomers [67], but separations remained poor in general, with badly shaped peaks, while very large volumes of methanol as eluent were required. The problems were even greater when species with more than one double bond had to be eluted, since they were held very strongly in the column.
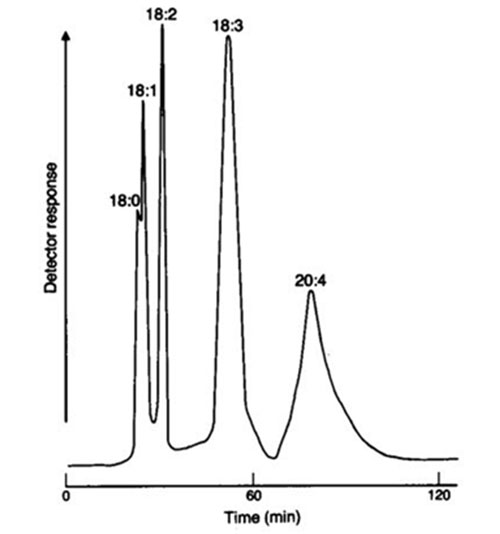
Other resins were tested in order to improve resolution and to shorten the duration of the runs. The commercial Amberlyst XE 284™ [174] and Amberlyst XN1010™ [66] were found to be better, and partial loading of the columns with silver ions also helped [6,7,9]. However, the results improved significantly only when the mobile phase of methanol was modified with acetonitrile [59], which enabled a reasonable resolution of monoenes, dienes, trienes and tetraenes (Fig. 20). Better results in fatty acid separations were obtained also by grinding the resins first to 100-200 mesh [174] and then to 270-350 mesh [171]. Saturated, trans-18:1 and cis-18:1 FAME were clearly separated at ambient temperature by isocratic elution with methanol on Amberlyst XE284™ impregnated with silver ions (refractive index detection) [171]. A temperature gradient from 25 to 60°C (gradient rate not stated) allowed the separation of linolenic acid isomers differing in the configuration of the double bonds (ttt, ctt, cct, ccc) on the same column [171]. Recently, Adlof reported a preparative separation of intact egg and soybean phosphatidylcholines by silver resin chromatography [5]. The analysis was performed on an Amberlyst XN1010™ (400 mesh) column fully converted into the silver form (ca. 36% w/w, silver/resin) with a solvent gradient of 100% methanol (held initially for 5 minutes) to 25% acetonitrile in methanol over 40 minutes. The resolution was not perfect and some mixed fractions, such as 18:1-18:2 plus Sat-20:3 or Sat-20:4 plus 18:1-Tetraene plus Sat-22:6 (Sat = saturated fatty acids, Tetraene = tetraenoic fatty acids of unspecified chain length) were obtained.
Figure 20. Preparative separation of methyl stearate, oleate, linoleate, linolenate and arachidonate on silver-loaded macroreticular ion-exchange resin XN1010™ with refractive index detection [59]. The mobile phase was 20% acetonitrile in acetone at a flow-rate of 7.2 mL/min. (Reproduced by kind permission of the authors and of the Journal of the American Oil Chemists' Society, and redrawn from the original.)
The macroreticular resin columns could be used almost indefinitely without loss of resolution or sample capacity. In fact, the sample size could be quite large (4 to 14 g, for example [4,8]), since the surface area was very great and nearly all of the sulfonic acid groups were converted into the Ag+-form [4]. However, even after the last improvements, the volume of solvent required remained too large and the elution time was very long, while the resolution could not compete with that achieved by other silver ion chromatographic techniques. Thus, silver-loaded resins in low-pressure columns have found a limited application for medium- and large-scale preparative separations and then apparently only in the laboratory that developed them.
A recent and useful development of silver ion chromatography with low-pressure columns is the use of silver-loaded solid-phase extraction (SPE) columns in the analysis of lipids. These commercially pre-packed columns are available with many different packing materials, one of which is a silica-based ion exchanger, similar to those used for HPLC columns. Two communications appeared independently in 1989 [46] and 1990 [192], both dealing with the fractionation of fatty acid methyl esters.
The impregnation procedure with silver ions was simple and similar to that for analogous HPLC columns. For example, a solution of silver nitrate (20 mg) in acetonitrile-water (9:1, v/v; 0.25 mL) was allowed to percolate through a Bond Elut SCX™ SPE column wrapped in aluminium foil [46]. The column was then flushed sequentially with acetonitrile (5 mL), acetone (5 mL) and dichloromethane (10 mL), when it was ready for use. A similar procedure was applied to load a Chromabond SA™ SPE column [192], except that the washing solvents in this instance were methanol, dichloromethane and hexane. Proper conditioning of the column appeared to be important, but with care pure fractions (not contaminated with silver ions) could be obtained [46]. For complex mixtures, sample loads of no more than 0.5 mg were recommended [46,95].
Fatty acid methyl esters with up to six double bonds were resolved on the Bond Elut™ column, with stepwise elution with dichloromethane, acetone and acetonitrile in suitable proportions [46]. In fractions with zero to three double bonds, there was almost no cross-contamination, and only 5% cross-contamination was observed for later fractions in complex samples which contained unsaturated fatty acids with a wide range of chain lengths [46]. The procedure described by Christie [46] is simple and easy to perform, giving good results even with complex fatty acid mixtures of marine (algae and invertebrates) origin [142], and it can be recommended as a preliminary step in GC or GC-MS analysis of unknown fatty acid samples.
Christie adapted the technique for the fractionation of triacylglycerols [48], and it has also been used for cholesterol esters [95]. With relatively saturated triacylglycerols such as those from cocoa butter, palm oil and sheep adipose tissue, SSS and SSM species were each obtained as distinct fractions, SMM and SSD eluted together, while the more unsaturated species were eluted as one fraction, by stepwise elution with appropriate mixtures of dichloromethane, methyl acetate, acetone and acetonitrile [48]. The fractionation of cholesterol esters required the same elution scheme as was used for fatty acid methyl esters, and plasma cholesterol esters with up to six double bonds in the fatty acid moiety were successfully resolved with less than 5% cross-contamination [95]. Such procedures have been reviewed by W.W. Christie in Advances in Lipid Methodology - One .
It is evident that low-pressure silver ion column chromatography has never had a leading role in the analysis of lipids, and it has been used most often for relatively large-scale fractionation or purification of lipids species. Now, even this aspect of column chromatography is losing its importance, since other techniques have been developed that are more effective and easier to handle. Moreover, modern chromatographic and spectroscopic methods for structural elucidation usually require only a few milligrams of material.
F. Combined Chromatographic Techniques
It is now widely accepted that there is no single method of analysis that is capable of the complete resolution of a complex mixture of natural or modified lipids [114]. A properly chosen sequence of chromatographic separations provides much more detailed and unambiguous information on sample composition. The strategy of glycerolipid separation and determination by using complementary analytical techniques is thoroughly discussed in the excellent review by Kuksis et al. [114]. Among the chromatographic techniques available, silver ion chromatography has a key position in that it separates lipids on the basis of a single molecular property - degree of unsaturation. Subsequent fractionation by high-temperature GC or reversed-phase TLC or HPLC then separates mainly on the basis of the chain lengths of the components. Therefore, silver ion chromatography is probably most useful as the first preparative step in an analysis. What should be recognized, however, is that the final results can only be as complete, precise and accurate as this preliminary separation.
The combination of silver ion TLC with GC is the oldest and most widely used form of such sequential techniques and is still invaluable for the analysis of complex mixtures of fatty acids [16,204], including geometrical [124,136], and positional [157,158] isomers. Silver ion HPLC in conjunction with GC and GC-MS appears especially useful for detailed analysis of complex fatty acid mixtures in unknown samples of marine [52], animal [3] and plant [51] origin. The combination of silver ion TLC with high temperature GC, initially on nonpolar phases but now more often on polarizable phases in capillary columns of fused silica, has been widely used for the analysis of molecular species of phospholipids [114]. Phosphatidylcholines, for example, can be hydrolysed first to diacylglycerols and then converted to diacylglycerol acetates [113] or tert-butyldimethylsilyl ethers [139]. After separation by silver ion TLC, these derivatives can be directly subjected to GC analysis [114,139] (see also Section C.4). Silver ion TLC and high-temperature GC have also been employed for the analysis of triacylglycerols [121], and most of the present knowledge of the triacylglycerol structure of a great number of natural fats and oils is based on the results obtained by this combination of methods. A recent example is the analysis of milk fat triacylglycerols [122] where silver ion TLC was combined with capillary GC.
A detailed investigation of Baltic herring triacylglycerols has been reported recently, in which silver ion TLC was followed by supercritical fluid chromatography [103].
The value of complementary separations of triacylglycerols by silver ion and reversed-phase chromatography was recognized long ago [121]. The sequential application, for example, of silver ion TLC and reversed-phase TLC (RP-TLC) in the analysis of some seed oils [11,35,200,201] provided the most detailed direct information on the triacylglycerol structure available before the combination of silver ion HPLC and RP-HPLC was introduced into lipid analysis. The former should still be considered as an alternative to the more sophisticated but expensive HPLC technique. Reversed-phase TLC was originally a messy technique that was not easy to control, but modern methodology does not suffer from such drawbacks [11,35].
RP-HPLC separations of triacylglycerols and phospholipid derivatives are now very efficient and straightforward [44], but it has been noted that certain natural mixtures are more effectively resolved after first being fractionated by silver ion TLC. Some excellent examples of such separations of cottonseed [23], soybean [198], fish [199] and evening primrose oils [159], and benzoyl derivatives of some phospholipids [25,179] have been reported. Recently, silver ion TLC was successfully replaced by silver ion HPLC as a preliminary to separation by reversed-phase HPLC in the analysis of palm oil and cocoa butter [188], fish oils [116] and meadowfoam oil [144]. The possibility of applying complex gradient elution procedures in both stages of the analysis has greatly enlarged the resolving power of these separations, and has sometimes allowed single molecular species to be correctly determined even when present in minor quantities.
Abbreviations
FAME, fatty acid methyl esters; FID, flame-ionization detector; GC, gas chromatography; HPLC, high-performance liquid chromatography; MS, mass spectrometry; RP, reversed-phase; SPE, solid-phase extraction; TLC, thin-layer chromatography. With molecular species: S, M, D, T, Te, P and H denote saturated, monoenoic, dienoic, trienoic, tetraenoic, pentaenoic and hexaenoic acyl moieties, respectively.
Nikolova-Damyanova, B. Silver ion chromatography and lipids. In: Advances in Lipid Methodology - One. pp. 181-237 (1992) (Ed. W.W. Christie, Oily Press, Ayr). Published here by kind permission of P.J. Barnes & Associates (The Oily Press), who retain the copyright.