Silver Ion Chromatography and Lipids, Part 3
The Author: Boryana Nikolova-Damyanova, Institute of Organic Chemistry, Centre of Phytochemistry, Sofia 1113, Bulgaria
- A. Introduction
- B. Principles of Silver Ion Complexation with Double Bonds
- C. Silver Ion Thin-Layer Chromatography
- D. Silver Ion High-Performance Liquid Chromatography
- E. Low-Pressure Silver Ion Column Chromatography
- F. Combined Chromatographic Techniques
- G. References
1. Some Practical Considerations
In recent years, HPLC has become one of the most widely applied separation techniques in lipid analysis, as documented by Christie [44]. After a timid start because of detection problems, HPLC was developed rapidly when new universal detectors suited to lipids became available. There is probably no separation that has been achieved by TLC, which could not be repeated by HPLC, often with greater convenience and speed. Until recently, separation of lipids by HPLC in the reversed-phase mode had no rival, but silver ion HPLC has now become an established technique. It is possible to wonder whether separations in the silver ion mode are indeed necessary when reversed-phase HPLC separates lipids according to both chain length and degree of unsaturation. The problem lies in the fact that the dual nature of the latter separation process can hamper the analysis of real samples; the number of components is often so great that identification becomes difficult or even impossible. On the other hand, there is considerable evidence that the combination of silver ion and reversed-phase separations provides information that cannot be achieved by any of these when applied on its own (see Section F).
The main problem with silver ion HPLC has been the development of a stable and reproducible system with a controlled silver content and reasonable operational life. The most important part in such a system is the column, which can be prepared in two general ways, i.e. by impregnating a support (usually silica gel) with a silver salt or by binding the silver ions to an ion-exchange medium. Alternatively, it is possible to add a silver salt to the mobile phase during a conventional reversed-phase HPLC separation.
Many of the separations performed so far have employed columns packed in the laboratory with silica gel impregnated with silver nitrate. Generally, the coating procedure involves dissolving the required amount of silver nitrate in a suitable solvent (methanol [87], acetonitrile [88] or water [22]) in a round-bottomed flask protected from light; the sorbent is either added directly to this solution [22]) or is first suspended in acetonitrile [87]. After evaporating the solvents on a rotary evaporator under reduced pressure, the material is ready to be packed into the column, usually by a procedure proposed by Heath et al. [87]. The column is stored with hexane as mobile phase. Of the different commercial silica gels tried, those with the smallest particle size were found to give the best results [83]. The recommended level of silver nitrate in the column was 5 to 10% (w/w) [183]. Although higher proportions did not affect the resolution significantly, the contribution of the adsorption activity of the silica surface to the retention mechanism increased when the silver content fell below 5%; this was considered to be the main reason for the pronounced peak tailing observed in such cases [183].
Such columns are not available commercially, and their preparation requires much practice and skill [83]. Therefore, separations can seldom be repeated in another laboratory without some modification. In an attempt to simplify the packing problem in laboratories with no access to specialised equipment, Aitzetmuller and Goncalves [10] described a method for impregnation of a commercial silica column in situ, in which a solution of silver nitrate in acetonitrile was injected as an immiscible plug while heptane was pumped continuously. While resolution of methyl oleate and methyl elaidate was achieved with this column, no further reports of its use have appeared.
The main disadvantage of columns containing an adsorbent impregnated with silver nitrate is that the silver ions bleed from them in use and their working life is limited. This can be overcome in part by using a pre-column of silver nitrate, but plugging of the column or precipitation of silver nitrate is also possible. The leached silver nitrate is corrosive and has the potential to damage the detector systems, and of course it is a contaminant of fractions collected when the technique is used in a preparative mode.
Silver ions are held much more strongly by the support when they are attached to a cation exchanger. Two main types of support have been employed so far, i.e. macroreticular sulfonic acid resins and silica gel with chemically bonded phenylsulfonic acid groups.
The utilization of macroreticular resins is discussed in Section E, although there is no distinct difference in the instrumentation used, except that somewhat lower pressure is applied to the column because of the larger particle size of the sorbent. In some of the recent applications [5,174], the resins were first ground to a particle size compatible with the HPLC equipment and the experimental conditions were very similar to those of HPLC. Separations on a silver-loaded aluminosilicate support have also been described [118]. Although this support was used successfully in an HPLC column to separate p-bromophenacyl esters of isomeric C16 and C18 fatty acids, no further applications were described and it may be that the preparation of the column is too laborious and irreproducible.
The use of pre-packed cation-exchange columns, containing benzenesulfonic acid chemically bonded to silica gel, appears to be the most advantageous approach. Such columns are supplied either in the H+ or Na+ form, and the former must first be neutralized (by flushing sodium or ammonium hydroxides through the column) before being treated with aqueous silver nitrate. In one published procedure [155], 1M silver nitrate (150 mL) was pumped through a RSilCAT™ (5 µ particles) column, followed by large volumes of water, methanol, acetone, ethyl acetate, chloroform and hexane. However, a simpler approach [43] consisted in injecting 20% aqueous silver nitrate (1 mL) via the Rheodyne™ injector (in 50 µL aliquots at 1 minute intervals) into an aqueous mobile phase in a Nucleosil™ 5SA column. The column was then washed with methanol for 1 hour and for a further hour with 1,2-dichloroethane.
Such columns were reported to be stable for long periods of time, although the resolutions deteriorated slowly. When this became troublesome, it was recommended [43] that the column be flushed with methanol-acetonitrile, and if this did not help - to repeat the silver ion loading procedure. The resolutions achieved with these columns were excellent and no leaching of silver ions occurred, a property that is of special value in preparative applications. To date, these columns have been used in a few laboratories only, and there is little published information on how the loading procedure will work in other hands. However, a commercial silver ion column of this type is now available from Chrompack Ltd. (Middelburg, Netherlands – now part of the Varian group). A column can last for up to a year of careful use; although retention times diminish slowly, this can be compensated for by reducing the polarity of the mobile phase [50]. Oxidized lipids should not be analysed and ethers should not be used in the mobile phase, since traces of hydroperoxides can react irreversibly with the silver ions. The actual silver ion content of such columns is not known, but is no more than 80 mg [43]; it depends on the number of sulfonic acid groups bound to the silica support and on the degree of replacement of the initial cation with Ag+. Although the properties of columns from different manufacturers may vary somewhat, it is a simple matter to modify mobile phases to suit particular analyses. The advantages of the columns are such that they will certainly find much wider use in HPLC.
It is possible to perform a separation in the silver ion mode by adding the silver salt to the mobile phase during a conventional reversed-phase HPLC separation. For example, aqueous silver nitrate and silver perchlorate have been added to isopropanol [175] or methanol [33] to form the mobile phase. Although the selectivity of the separation is changed appreciably by this means in comparison to that in the reversed-phase mode alone, the disadvantages of using a corrosive mobile phase of this kind are such that the procedure has found little favour.
HPLC separations rely very much on the detection system available, and this can restrict the choice of mobile phase and in turn the selectivity of separation. In theory, almost any type of detectors can be used in silver ion HPLC of lipids, although each has its limitations [44,203] (see also the review by W.W. Christie in the same volume ...). Ultraviolet spectrophotometric detectors are the most widely used in HPLC, but with the technique in the silver ion mode they tend to be restricted to specific derivatives of lipids that absorb above 235 nm. For example, fatty acids can be converted to p-bromophenacyl esters instead of methyl esters; and diacylglycerols, derived from phospholipids, can be analysed as benzoates rather than acetates. The problems with fluorescence and infrared detectors are similar and they have not been used in silver ion HPLC so far. The differential refractometer or refractive index detector is also common in laboratories and was utilized in many of the first published separations with silver ion HPLC. Again, they have serious disadvantages, and their future use will certainly diminish because of their unsuitability for gradient elution especially.
Recently, an increasing use has been made of evaporative light-scattering (“mass”) detectors in HPLC. These are universal in their applicability, give excellent results under gradient elution conditions and are simple to operate. They are not affected by changes in ambient temperature, or small variations in the flow-rate of the mobile phase, and the only essential requirement for the solvents is that they must be sufficiently volatile. Although these detectors are destructive, a stream-splitter can be inserted between the column and the detector in order to permit the collection of fractions for further analysis. The newer commercial models possess high sensitivity, and although only a part of the eluent passes through the system, the response is sufficient for many purposes and is reproducible. One disadvantage of these detectors is the need for dry, filtered compressed air in large amounts, so an air-compressor must be installed. Similarly, transport-flame ionization detectors have appreciable potential for lipid analysis in general and in the silver ion mode in particular, but this has still to be fully realized.
The proper choice of solvents for use in the mobile phase is of great importance. Although theoretical principles of selectivity have been developed [44,166,184], most analysts have chosen the solvents empirically from the experience gained by silver ion TLC, especially with the approach in which silica gel adsorbents impregnated with silver nitrate were employed. The nature of the interaction between silver ions, the unsaturated analytes and different mobile phases is less well understood for cation-exchange columns loaded with silver ions. Chlorinated solvents appear to be particularly suitable, and acetonitrile, which complexes strongly with silver ions displacing unsaturated solutes, is of special value in this form of silver ion HPLC. For optimum resolution, a linear or concave gradient with increasing proportion of the more polar solvent in the mobile phase has been recommended with triacylglycerols [83].
As with silver ion TLC, the retention of lipids on comparable HPLC systems has been ascribed to a mixed interaction mechanism [118,183], partly based on silver ion complexation and partly on the interaction of polar groups of the solute with unreacted polar groups of the support (silanol or sulfonic acid groups or both). Solvents like benzene, toluene and acetonitrile are assumed to interact predominantly with the silver ions, reducing the interaction with the double bonds [88,156]. In contrast, methanol, isopropanol and acetone may act by reducing the strength of the interaction with the silanol and other polar moieties [155]. These properties can be manipulated to effect highly selective resolutions of species differing in the number, configuration and position of double bonds in lipids (see for example [53]). Of course, solvents must be of high purity and HPLC grades should be used (or they should be distilled before use), and they must not interact in undesirable ways with the support or with the solute.
A development of a related technique was reported recently. Capillary columns home-packed with the Nucleosil 5SA™ and loaded with silver ions have been used with a supercritical fluid as the mobile phase (carbon dioxide-acetonitrile-acetone, 94.3:5.3:0.4 by volume) to separate triacylglycerols from seed oils [62,63] (see also the review by Paivi Laakso, also published in Advances in Lipid Methodology - One). It is too early to predict the future development of this technique, but for the moment conventional HPLC affords better resolution and is more flexible.
2. Separation of Fatty Acid Derivatives
Since the separation principle for silver ion HPLC is the same as for TLC, the elution order is expected to be similar to TLC when the silver ions are held by the support and opposite when silver is introduced into the mobile phase (see Section D.1 above). With HPLC supports impregnated with silver nitrate, a sinusoidal relationship might be expected between the retention volume and the position of the double bond in isomeric fatty acids. A fatty acid with an acetylenic bond will elute just ahead of a cis-monoene, trans,trans-non-conjugated dienes and cis,trans-conjugated dienes will elute with cis-monoenes, and longer-chain fatty acids will be retained less strongly than the corresponding C18 isomers. Some of these relationships have been observed experimentally [137].
As already stated, there are three general ways to introduce silver into the HPLC system and all have been applied to resolve fatty acid derivatives. Good resolution of C18 fatty acids with zero to two double bonds was achieved on a column of Partisil 20™ impregnated with 2% silver nitrate, with a mobile phase of 1% tetrahydrofuran in hexane [22], and near base-line separation of trans-9-18:1 from cis-9-18:1 was achieved in less than 5 minutes. By changing the sorbent to Spherisorb S5W™ and increasing the silver nitrate content to 5%, the same authors separated fatty acid methyl esters from margarine into groups containing either trans- or cis-positional isomers. Within the groups, components were only partially resolved and were probably not identified correctly. Resolutions of the geometrical isomers of 9,12- and 9,15-18:2 were performed on a laboratory-packed Biosil A™ column impregnated with 20% silver nitrate. However, the author presented only the retention volumes and not the chromatograms; hence one cannot see the actual resolution. From the data presented it is clear that the two series of isomers would overlap if analysed together under the conditions described, i.e. isocratic elution with benzene [170]. Methyl linolenate could not be eluted from this column.
With a column prepared in a similar way but with a mobile phase of 0.4% acetonitrile in hexane, Ozcimder and Hammers [149] separated fatty acid derivatives with three to six double bonds from cod liver oil. The more saturated components were poorly resolved because of their high content in the sample. A particularly good separation of hydroxyoctadecadienoates was achieved on a laboratory-prepared column [84], where the species were resolved according to the position of the hydroxyl group in addition to the configurations of the double bonds, thus demonstrating the effect of the mixed retention mechanism in silver ion silica HPLC. An analyst who has experience in preparing his own columns may find that this approach is more versatile in that the silver content can be controlled and changed when needed. On the other hand, as in TLC, the preparation of the column needs some practice and skill, and resolutions can rarely be repeated in another laboratory in exactly the same way. The operational life of columns packed with silica impregnated with silver nitrate is short, and those used to analyse fatty acids allowed only 20 [22] to 50 runs [170] because of leaching of the silver ions.
Silica-based ion-exchangers loaded with silver nitrate, as described above (see Section D.1 above), provide a more complete and reproducible resolution of fatty acids [43,155]. By using a RSilCAT™ (5 µm) column, and a mobile phase of chloroform-methanol-acetonitrile-acetic acid (89.5:8:2:0.5 by volume), Powell [155] was able to achieve a base-line separation of a mixture of 14C-labelled eicosenoic and monohydroxyeicosenoic acids (radioactivity monitor as detector).
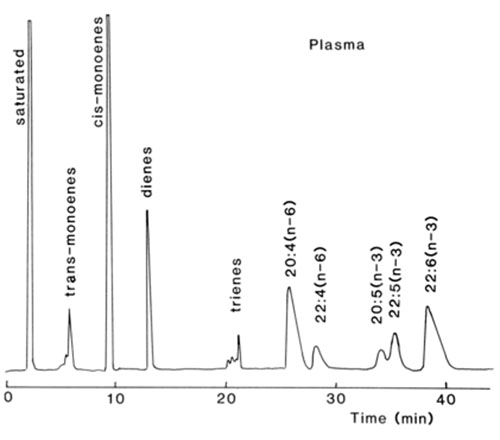
Perhaps the best separations of fatty acids with zero to six double bonds to have been achieved so far were obtained with the silica gel-based ion-exchanger Nucleosil 5SA™ in the silver ion form [43,52] (Fig. 13). Components were eluted with a linear gradient of methanol to methanol-acetonitrile (9:1, v/v) for about 30 minutes at a flow-rate of 0.75 mL/min. Subsequently, better resolution was obtained with a modified system and tetraenes were separated into (n-6) and (n-3) fractions, eluting in this order [52]. Even more remarkable resolutions were achieved on the same column by isocratic elution of phenacyl ester derivatives of fatty acids with 1,2-dichloroethane-dichloromethane (1:1, v/v) as the mobile phase [53].
Figure 13. Separation of fatty acid methyl esters from plasma lipids by silver ion HPLC with evaporative light-scattering detection [47]. A Nucleosil 5SA™ was loaded with silver ions as described in the text. The mobile phase was a gradient of 1,2-dichloroethane-dichloromethane (1:1, v/v) to 1,2-dichloroethane-dichloromethane-methanol-acetone (45:45:5:5 by volume) over 40 min. at a flow-rate 1.5 mL/min.
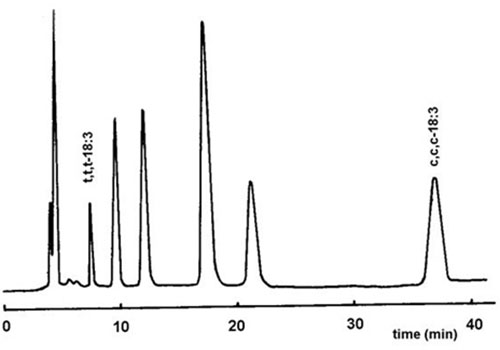
In addition to the resolution of cis/trans isomers, appreciable fractionation according to the positions of the double bonds was possible. Thus, trans-11-, trans-9-, cis-11- and cis-9-18:1, as a standard mixture, were clearly resolved. Substantial but incomplete resolution of the complex mixture of esters in a hydrogenated oil was possible, but GC-MS data showed that the order of elution of isomers followed the general pattern established for silver ion TLC, i.e. 12- and 13-isomers were less strongly retained than 7- and 8-isomers for both the cis and trans configuration of the double bond. Similarly, excellent resolution of geometrical isomers of linoleic and linolenic acids was achieved with a mobile phase of 1,2-dichloroethane-dichloromethane-acetonitrile (49.75:49.75:0.5 by volume) in only 10 and 40 minutes, respectively. Although the isomers were not identified, it was presumed that the elution order for the linoleate isomers was t,t > t,c/c,t > c,c, for example (Fig. 14). The potential of the method for the determination of the trans-monoene content in several hydrogenated oils and fats was explored [53]. Phenacyl esters of fatty acids have the advantage of absorbing in the UV region, permitting the use of a UV detector for quantification of the components. A similar column with a gradient of dichloroethane-dichloromethane (1:1, v/v) to dichloroethane-dichloromethane-acetonitrile-methanol (40:40:1:1 by volume) was employed to isolate specific fatty acid fractions from seed oils containing cyclopentenyl fatty acids [51].
Figure 14. Separation of the geometrical isomers of linolenic acid, as phenacyl esters, by silver ion HPLC [53]. The column was as in Figure 13 but the temperature was maintained at 38°C. Isocratic elution with 1,2-dichloroethane-dichloromethane-acetonitrile (49.75:49.75:0.5 by volume) at a flow-rate of 0.75 mL/min was used. Species were detected at 242 nm. (Reproduced by the kind permission of the authors and of the Journal of Chromatography, and redrawn from the original).
A semi-preparative SCR-101H™ ion-exchange column (particle size 10 µ), prepared as described above [43], was utilized for the separation of very-long-chain polyunsaturated fatty acids [164]. The fatty acids as their methyl esters were resolved by a linear gradient of methanol to methanol-acetonitrile (1:1, v/v) over 30 minutes with UV detection at 208 nm. While the separation was not perfect, fractions enriched in tetra-, penta-, hexa- and heptaenoic species were isolated. Picolinyl ester derivatives of these fatty acids were also resolved on this column with a gradient in the mobile phase from hexane-dioxane-isopropanol (40:10:50 by volume) to dioxane-isopropanol (50:50, v/v) over 20 minutes and then to isopropanol alone over a further 25 minutes, and with UV detection at 266 nm [163]. The last separation may be of particular value, as picolinyl esters can be used directly for structure determination by GC-MS (see the review by Harvey, also in Advances in Lipid Methodology - One).
The high selectivity of silver ion HPLC with ion-exchange columns has been demonstrated by applications to stereochemical analysis of some hydroxy-eicosatrienoic [85] and eicosatetraenoic [55] fatty acids, as reviewed by Christie (also in Advances in Lipid Methodology – One).
Fatty acid methyl esters have also been resolved by introducing a silver salt into the mobile phase during a reversed-phase HPLC separation. One of the first examples was for the separation of methyl oleate and methyl elaidate on a LiChrosorb RP8™ column with 1.5% silver nitrate in isopropanol-water (5:4, v/v) as mobile phase [175]. This approach has limited value, because the results are not better than with other silver ion techniques and because of potentially deleterious effects of silver ions on the HPLC equipment. Now that easy methods for the preparation of stable silver columns for HPLC are available, the technique will find much wider application as an analytical and a micropreparative technique able to provide pure fatty acid fractions in a form suitable for further analysis.
3. Molecular Species of Triacylglycerols
Silver ion TLC has been of such value for the analysis of triacylglycerols that it was inevitable that HPLC would be used in a similar way. The first separations described were performed with reversed-phase columns and mobile phases containing silver ions. However, the elution patterns obtained were complicated and such methods did not appear to offer a significant advantage over conventional reversed-phase separations [44].
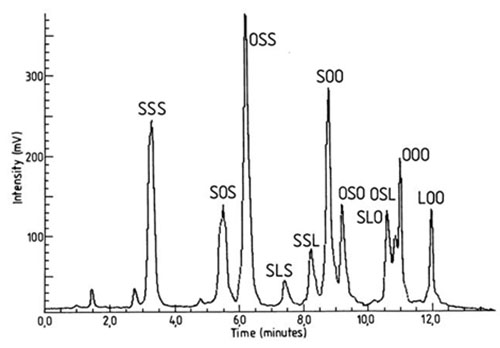
Better results were obtained with columns of silica gel, e.g. Partisil 5™ [82,183], Spherosil XOA™ [130] and Nucleosil 100-3™ [101], impregnated with 10% (w/w) silver nitrate, and prepared as described in Section D.1 above. Saturated and mono-, di- and triunsaturated fractions were resolved by elution with benzene at 6.8°C [183]. Moreover, the monounsaturated fraction was resolved into two subfractions corresponding to the two positional isomers SSO and SOS (S = saturated, O = oleic acid). Subsequently, the separation was greatly improved and the elution time was shortened when 3-micron silica and a gradient in the mobile phase were employed to resolve triacylglycerol samples [101]. Two solvent mixtures, toluene-hexane (1:1, v/v) and toluene-ethyl acetate (9:1, v/v), were combined in a linear gradient (with transport-flame ionization detection) to provide the best resolution of positional isomers of triacylglycerols, i.e. SOS-OSS, SLS-SSL, SOO-SOS and SLO-OSL (L = linoleate), achieved to date by silver ion HPLC (Fig. 15).
Figure 15. Separation of positional isomers of triacylglycerols by silver ion HPLC on 3-micron silica (Nucleosil 100-3™) column impregnated with 10% (w/w) silver nitrate [101]. The mobile phase was a multilinear combination of two solvent mixtures - A, toluene-hexane (1:1, v/v), and B, toluene-ethyl acetate (9:1, v/v) at a flow-rate of 1.5 mL/min. The components were detected by transport-flame ionization detection. S, O and L denote saturated, oleic and linoleic fatty acyl residues respectively. (Reproduced by kind permission of the author and of the Journal of the American Oil Chemists' Society, and redrawn from the original.)
The separation on columns of this type is, however, still limited to relatively simple samples with a low concentration of dienoic fatty acyl groups, while silver ions inevitably bleed continuously from the column. An appreciable step forward was made when the silica-based ion-exchange column in the silver ion form was utilized to separate triacylglycerols [43] (see also Sections D.1 and D.2 above). Palm oil triacylglycerols were first resolved at ambient temperature with a linear gradient of 1,2-dichloroethane to acetone over 30 minutes at a flow-rate of 0.75 mL/min, and with evaporative light-scattering detection. Excellent separation of triacylglycerols with zero to three double bonds was achieved with no cross-contamination and no detectable silver ions in the fractions. Dichloroethane and acetone were preferred as solvents instead of methanol, which had earlier been used with ion-exchangers, as there was some danger that residual sulfonic groups might catalyse the transesterification of triacylglycerols in the presence of methanol [6].
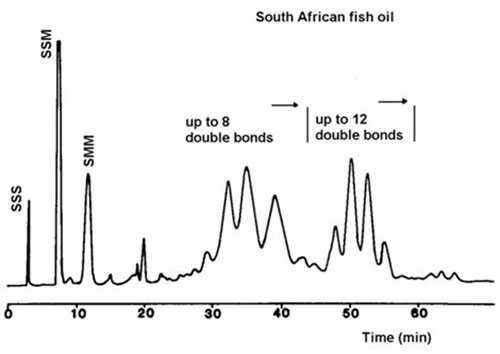
More complicated elution schemes were employed to resolve complex triacylglycerol mixtures of greater complexity, such as those from rat adipose tissue, sunflower oil [45], linseed oil [45], fish oils [45,116,117], evening primrose oil [49] and meadowfoam oil [144]. Three solvent mixtures were generally utilized to establish gradients in the mobile phase, i.e. (A) 1,2-dichloroethane-dichloromethane (1:1, v/v), (B) acetone and (C) acetone-acetonitrile in proportions from 9:1 to 2:1 (v/v) [45]. For example, a gradient of A to B generated over 40 minutes was sufficient to resolve triacylglycerols with up to three double bonds in total, including the distinctive separation of species containing oleic acid from those containing elaidic acid [45]. A mixture of components with zero to nine double bonds was resolved in a single run with a gradient of 100% A to 50% A-50% B over 10 minutes, then to 70% B-30% C over 20 minutes and finally to 100% C (C = acetone-acetonitrile (4:1, v/v)) over a further 30 minutes [45,49]. An even more complicated gradient was employed to resolve fish oil triacylglycerols and although the separation of highly unsaturated species was far from being perfect, it was reproducible and appears to be the most detailed fractionation of its kind reported to date [45,116,117] (Fig. 16).
Figure 16. Separation of triacylglycerols from a fish oil of South African origin by silver ion HPLC with evaporative light-scattering detection [45]. The column was as in Figure 13 above. The solvents used were A, 1,2-dichloroethane-dichloromethane (1:1, v/v), B, acetone and C, acetone-acetonitrile (4:1, v/v) at a flow-rate of 1 mL/min. A linear gradient of A to 50% A-50% B over 10 min was changed to 70% B-30% C over another 20 min and finally to 100% C over a further 40 min. S and M denote saturated and monoenoic fatty acyl residues. (Reproduced by kind permission of the author and of the Journal of Chromatography, and redrawn from the original.)
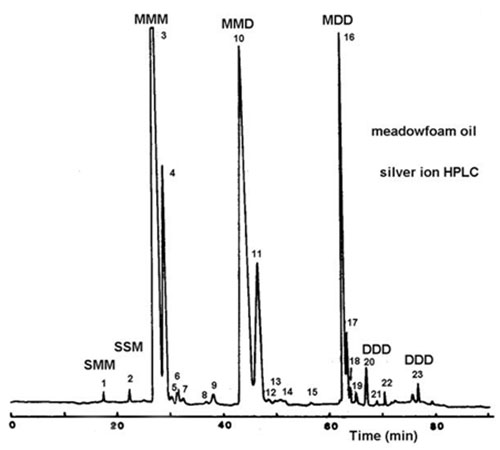
One dienoyl residue is retained more strongly then two monoenes in the same molecule and one triene (18:3(n-3)) is equivalent to two dienes. In general, the triacylglycerols migrate in an order comparable to that obtained by silver ion TLC. The latter sometimes resolves species that contain one linolenoyl from those containing two linoleoyl moieties [78,165], but clear separation is achieved under special conditions only [189,190]. The picture with the more highly unsaturated species found for example in fish oils [116,117] was too complicated to establish a clear migration order. Generally, triacylglycerols are held more strongly when double bonds are concentrated in one fatty acid moiety [117], and in some instances separation may be governed by the position of the double bond in one of the fatty acid moieties and indirectly by its chain length [144]. For example, the triacylglycerols of meadowfoam oil were fractionated into three main groups of peaks, representing the classes MMM, MMD and MDD, respectively. Within each group, distinct peaks were seen for species differing in the position of the double bond in only one fatty acyl moiety in the molecule, namely 11-20:1, 5-20:1, 13-22:1, 5-18:1 and 9-18:1, eluting in this order (Fig. 17).
Figure 17. Separation of triacylglycerols from meadowfoam seed oil by silver ion HPLC with evaporative light-scattering detection [144]. A linear gradient of three solvents was used at a flow-rate of 0.75 mL/min.; A, 1,2-dichloroethane-dichloromethane (1:1, v/v); B, acetone; C, acetone-acetonitrile (9:1, v/v), starting from 100% A and changed to 50% A-50% B over 50 min. and then to 50% B-50% C over a further 50 min. M and D denote monoenoic and dienoic fatty acyl residues. (Reproduced by kind permission of the Journal of the American Oil Chemists' Society.)
The separations discussed above demonstrate the great resolving power of the silver ion HPLC system coupled with evaporative light-scattering [43] or transport-flame ionization (FID) detectors [101] and with instrumentation for gradient elution. The possibility to use a complex solvent gradient makes this system highly selective and is perhaps the greatest advantage of silver ion HPLC over the other silver ion techniques employed to resolve triacylglycerols. A linear gradient has been used most often, although concave gradients are generally considered more effective [83]. The combination of chlorinated hydrocarbons with acetone and acetonitrile in the mobile phase appears to be suitable for resolution of a wide range of natural triacylglycerol mixtures [43,117]. Samples of 1-2 mg can be injected without significant loss of resolution. When a stream splitter is inserted between the column and the detector, clean fractions (uncontaminated with silver ions) can be collected and subjected to further analysis by RP-HPLC, GC or GC/MS. After gradient elution, the column must be well conditioned with the starting solvent before the next analysis.
The development of a reliable method for silver ion HPLC is of great importance for triacylglycerol analysis. Resolution is based on a single molecular property only and this simplifies the identification of components and their further analysis. Silver ion HPLC and RP-HPLC applied in a complementary way are to date the most powerful tools in the analysis of triacylglycerols (see Section F).
4. Molecular Species of Complex Lipids
Silver ion HPLC has had a limited use only for the analysis of molecular species of complex lipids. Most separations reported have been performed with HPLC in the reversed-phase mode. When this has given inadequate resolution of critical pairs, silver ion HPLC has occasionally been used instead. As with other lipids, the separation with silver ion HPLC is based on the total number of double bonds in the fatty acid moieties. It is generally considered that nonpolar derivatives of complex lipids are more cleanly resolved than are the intact compounds, but the effect appears to be less marked with those silver ion HPLC separations reported so far.
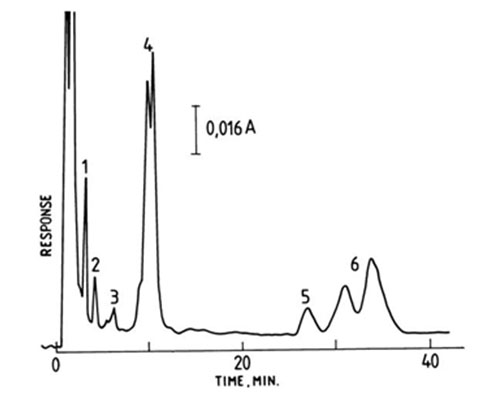
Benzoylated sphingomyelins of brain origin were resolved into saturated (mainly stearate) and monounsaturated (mainly 24:1) species on a commercial prepacked silver-loaded column, presumably of the ion-exchange type [102]. Fractions were eluted isocratically with methanol-isopropanol (8:2, v/v) for 50 minutes and detected by UV spectrophotometry at 230 nm. Individual phosphatidylcholines were resolved in a similar way with methanol as the mobile phase, and in this instance the column temperature was maintained at 45°C (Fig. 18). Species with up to six double bonds were separated. The more saturated components were only partially resolved while 18:0-22:6 and 16:0-22:6 (eluted in this order) formed two distinct peaks [102].
Figure 18. Separation of phosphatidylcholines from rat microsomes by silver ion HPLC with methanol as eluting solvent at a flow-rate of 2 mL/min and detection at 205 nm [102]. The temperature of the column was maintained at 45°C. The numbers near each peak represent the total number of double bonds in the species. (Reproduced by kind permission of the authors and of Raven Press, and redrawn from the original.)
Intact monogalactosyldiacylglycerols were separated on a C8 reversed-phase column with silver perchlorate (8 x 10-3 M) added to the mobile phase of methanol-water (9:1, v/v) [60,193]. Components with the same total number of double bonds were resolved, i.e. 18:2-16:4 from 18:3-16:3 and 18:2-16:3 from 18:3-16:2 (eluting in this order) [60]. This was consistent with other observations that silver complexation is stronger with diminishing chain length in a fatty acyl residue with a given number of double bonds. It will be worthwhile to experiment with silica-based ion-exchange columns in the silver ion mode. These columns, with appropriate solvent gradients and evaporative light-scattering detection, have the potential to greatly improve the separation of molecular species of complex lipids. There is also the possibility of preparing and separating suitable UV-absorbing derivatives, since this would enable direct quantification of the separated components by spectrophotometry.
5. Quantification
There are two general approaches to quantify species separated by HPLC and both can be used with the technique in the silver ion mode. Obviously, quantitative results are obtained with greatest convenience and speed when the signals of the detector can be used directly. Accurate measurement of peak areas is very important, but it must be recognised that detectors respond to lipids in different ways and must be carefully calibrated before quantitative work is undertaken. The properties of specific HPLC detectors have been reviewed elsewhere ([44], and see the review by Christie in Advances in Lipid Methodology - One.). Both external and internal standards can be used. To calibrate with external standards, equal volumes of samples which contain one or more components in known amounts are injected onto the column. A plot of peak area against concentration is constructed for each component within the concentration range expected in the natural samples; the calibration is considered valid when the plot is linear and passes through the origin.
A calibration of this type is especially suitable for fatty acid and diacylglycerol derivatives with UV-absorbing or fluorescent reagents, since the detector signal then comes from the derivatizing group only and is independent of lipid structure. An example of this approach was the determination of the trans-monoenoic fatty acid content of margarines, cooking fats and hydrogenated oils [53]. Fatty acids were converted to their phenacyl esters, and the calibration plot was linear in the range zero to 200 µg. On the other hand, when molecular species of intact lipids are separated, calibration with an external standard is a laborious operation. In addition, only a limited range of molecular species are available as commercial standards and others may have to be prepared from natural samples.
The internal standard technique requires a compound that is generically related to the species analysed. It should elute in the same region as the components to be determined but should be completely separated from them. At the earliest possible stage of the analysis, a precise amount must be added and carried through every step of the procedure, including extraction and derivatization. Finally, the response of the detector to each component of interest must be determined relative to that of the internal standard. For example, methyl santalbate (methyl octadec-9-yne-11-trans-enoate) was chosen as internal standard in the determination of triacylglycerols by refractive index detection [183]. Trilaurin was used successfully as an internal standard for the quantification of palm oil triacylglycerols with transport-FID detection [101]. However, the use of a short-chain triacylglycerol as an internal standard needs a highly selective separation system and cannot be applied when triacylglycerols with medium-chain or isomeric fatty acids are present.
One of the most widely used approaches to the quantification of molecular species separated by HPLC has been the indirect one. Detectors are used to monitor the resolution only, fractions are collected, the mobile phase is evaporated, the residue is dissolved in a suitable solvent and subjected to further analysis, most often GLC following transesterification. This permits identification of fractions as well as quantification. In principle, this method is identical to that employed with silver ion TLC as discussed in Section C.5. The triacylglycerol compositions of many fats and oils of plant, animal and fish origin, fractionated by silver ion HPLC, have been determined in this way [43,45,49,117,144].
Abbreviations
FAME, fatty acid methyl esters; FID, flame-ionization detector; GC, gas chromatography; HPLC, high-performance liquid chromatography; MS, mass spectrometry; RP, reversed-phase; SPE, solid-phase extraction; TLC, thin-layer chromatography. With molecular species: S, M, D, T, Te, P and H denote saturated, monoenoic, dienoic, trienoic, tetraenoic, pentaenoic and hexaenoic acyl moieties, respectively.
Nikolova-Damyanova, B. Silver ion chromatography and lipids. In: Advances in Lipid Methodology - One. pp. 181-237 (1992) (Ed. W.W. Christie, Oily Press, Ayr). Published here by kind permission of P.J. Barnes & Associates (The Oily Press), who retain the copyright.