Silver Ion Chromatography and Lipids, Part 2
The Author: Boryana Nikolova-Damyanova, Institute of Organic Chemistry, Centre of Phytochemistry, Sofia 1113, Bulgaria
- A. Introduction
- B. Principles of Silver Ion Complexation with Double Bonds
- C. Silver Ion Thin-Layer Chromatography
1. Some practical considerations
2. Separation of fatty acid derivatives
3. Separation of molecular species of triacylglycerols
4. Separations of molecular species of complex lipids
5. Quantification - D. Silver Ion High-Performance Liquid Chromatography
- E. Low-Pressure Silver Ion Column Chromatography
- F. Combined Chromatographic Techniques
- G. References
1. Some Practical Considerations
For many years, silver ion TLC has been one of the basic separation techniques employed in the analysis of lipids. TLC is rapid, simple and versatile and it does not require expensive instrumentation. The information obtained reflects the whole sample, thus helping the analyst to make rapid, correct and efficient judgements.
Both self-made and pre-coated plates have been used in silver ion TLC. Silica gel G, i.e. with calcium sulfate as binder (for fatty acid methyl esters and triacylglycerols), and silica gel H, i.e. without binder (for complex lipids), have most often been the supports. Recently, pre-coated plates with alumina impregnated with silver nitrate have been employed to separate fatty acid methyl esters with acceptable results [28], but alumina is known to react with some lipids and solvents [44] and it should be used with care.
In practice, the thickness of the adsorbent layer has been varied from 0.2 to 0.3 mm for analytical plates and from 0.5 to 1.0 mm for preparative plates. Fully automated spreaders are now available commercially, but simple spreaders are equally effective. Layers prepared in the laboratory are more versatile and should not darken when the plate is heated. However, some practice is needed for their preparation, and pre-coated plates are now often preferred.
There are two general ways to perform silver ion TLC of which by far the most common is to use a layer of adsorbent impregnated with a silver salt. While it is possible as an alternative to add silver salt to the mobile phase when a reversed-phase TLC separation is performed, this approach has found a limited use only in lipid analysis [148,160,197]. The silver salt, usually silver nitrate, is dissolved in water, methanol, ethanol, ammonia or acetonitrile in order to impregnate the layer. Although the influence of the salt anion has not been studied systematically, there is evidence that its nature may affect the resolution. For example, silver sulfamate [96] and silver benzenesulfonate [97] have been used in TLC adsorbents and were reported to give improved separations of fatty acid methyl esters. On the other hand, some exchange of anions with the calcium salt used as binder is inevitable, so the effect of the anion cannot be a simple one. An improved separation of methyl esters was observed when the plate was impregnated with an ammoniacal solution of silver nitrate [205], an effect ascribed to the formation of the Ag(NH3)2 complex.
Impregnation can be performed by incorporating the silver salt into the slurry of adsorbent used to make the layer, by immersing the prepared plate in a methanol, acetone [182] or acetonitrile [125] solution of the silver salt, or by spraying the plate with one of these solutions. However, the only method that affords proper control of the silver content of the layer is to add silver nitrate to the aqueous slurry of silica gel used to make the adsorbent layer. This is inconvenient and messy, and it is less used now. The content of silver nitrate in the layer may not in fact be critical, and immersion procedures can be standardized sufficiently well to provide satisfactory results. While spraying methods are sometimes used, they are less easily standardized; spraying may have to be repeated from two to six times until the adsorbent layer is properly wetted.
A “dynamic impregnation” technique [10] has recently been proposed in which the plate is developed with a 10 to 20% solution of silver nitrate in acetonitrile. The silver salt is absorbed into the layer in such a way that the silver content gradually decreases with increasing height. After a sample is applied, the plate is developed in the same direction; the gradient in silver content is reported to improve the separation of triacylglycerols, for example.
The silver content of the adsorbent layer has been varied between rather broad limits. Layers containing from 20 to 30% of silver nitrate (w/w) were considered necessary to achieve good resolution in some experiments [93,135,196,201], but this high percentage is very inconvenient in practice. For example, such plates are very sensitive to light and this can greatly hamper detection. Preparative TLC plates which contain from 5 to 20% silver nitrate in the adsorbent layer are now most often used. The immersion technique has been performed with 5 to 10% solutions of silver nitrate, while a 40% solution has been recommended for spraying [98], but the proportion of silver ions retained by the adsorbent was not determined with the latter technique. On the other hand, excellent results in the resolution of fatty acid methyl esters and triacylglycerols have been achieved on plates impregnated by dipping in only 0.5% methanolic silver nitrate [37,146]. Therefore, the silver content is only one of the factors to affect the resolution and should be considered in combination with all the other chromatographic conditions (see also Section B).
Plates prepared by the above procedures are usually activated prior to use by heating at 110°C for about one hour, but good results have been reporting after activation for 5 minutes only [37,146]. Thus, it seems that even the necessity for activation is questionable, and the analyst must trust to his own experience. This operation does seem to be very important for the silica gel H plates used to resolve polar lipids, when temperatures higher than 110°C for periods of much longer then one hour have been recommended [14]. For example, a distinctive resolution of molecular species of dimethyl phosphatidic acid derivatives was achieved on plates activated in three stages, i.e. at 50°C, at 80°C and finally at 110°C [107].
Atmospheric humidity was found to have an appreciable effect on separation, especially of highly unsaturated species [65]. It is recommended therefore that activated TLC plates be kept in a desiccator over drying agents (ideally in the dark). However, it is not easy to control humidity in practice, and this may be one of the reasons for the relatively poor overall reproducibility of Rf values in separations performed by silver ion TLC.
Most published separations have been performed in covered tanks in which the atmosphere has been saturated with the vapour of the mobile phase, and this saturation is considered to shorten the duration of development and often to improve the reproducibility. Again, there are no firm data to support this conclusion, and poor separation and tailing of zones have also been reported in these circumstances [93]. The author’s experience has been that better separations are obtained by using a container, the atmosphere of which is not saturated with mobile phase, or even an “open” container. The geometry and volume of the tank used for development can also affect the separation; it seems that narrow rectangular tanks and a moderate volume of the developing solvent provide better resolution [93].
Silver ion TLC plates are normally developed at ambient temperature, but the resolution of positional isomers of unsaturated fatty acids [135,146] or of triacylglycerols containing such acids [201] was possible only at temperatures of about -20°C. The stability of silver-double bond complexes increases when the temperature decreases [135], but this is not the only factor affecting the resolution. For example, the properties of the sorbent and mobile phase also change at low temperatures and the actions of all three factors probably enabled these fine separations.
Mobile phases usually consist of two or three component mixtures. Hexane or petroleum ether (b.p. 40-60°C), chloroform, benzene and toluene are most often the major components, while smaller proportions of diethyl ether, acetone, methanol, ethanol, or acetic acid may be added to these. Chloroform-methanol mixtures in proportions from 99:1 to 90:10 (v/v) seem to provide the best resolution of fatty acid methyl esters, triacylglycerols and dimethyl phosphatidic acid derivatives achieved so far. In addition, water or anhydrous acetic acid has sometimes been added in order to assist in the separation of highly unsaturated species [16,65].
Plates are often developed more than once to improve resolution. The separation should start with the most polar phase and proceed, after drying between runs, with mobile phases of gradually decreasing polarity. In this way, highly unsaturated components are resolved first and do not move further with subsequent developments when the more saturated components are separated. Obviously, the separation will gain much if a continuous development can be applied and a simple example has been described [37]; the development was allowed to proceed in an open cylindrical tank where a fixed volume (5 mL or more) of the mobile phase was added. As the mobile phase was eluted through the plate, it was permitted to evaporate from the upper edge. Resolution was very effective and excellent results have been reported [189,190], including the separation of positional isomers of triacylglycerols [145]. Although this “open” system is quite sensitive towards the laboratory environment, it operates very well in skilled hands.
In general, two approaches exist for the detection of lipids separated on silver ion TLC plates. Destructive procedures have been used for location, for identification to some extent and for quantification purposes, and they consist in carbonization of the separated species by heating after treatment with charring reagents. These can be introduced by spraying, by treatment of the plates with vapours or by incorporating the charring reagent into the layer. For example, up to 50% alcoholic solutions of sulfuric acid or phosphomolybdic acid have been used as spraying reagents [20,150]. Spraying is a rapid but inconvenient operation and should be avoided if possible. Reliable results were obtained by saturating the adsorbent layer with vapours of sulfuryl chloride [37]. Incorporation of the charring reagent into the layer should be performed with circumspection, since it may change the nature of the resolution. While this last approach to the detection of lipids has not been used often, successful experiments with phosphotungstic acid [13] and silver sulfamate [96] have been described.
For preparative purposes, developed plates are sprayed with a solution of a fluorescent indicator (e.g. 2,7-dichlorofluorescein, Rhodamine 6G) and viewed under UV light [19,20]. Zones are then carefully scraped from the plate and the lipids are extracted from the sorbent with suitable polar solvents, such as diethyl ether, methanol or chloroform. A mixture of chloroform and methanol is particularly effective and is suitable for highly unsaturated species, while water is added sometimes when extracting intact polar lipids. As such material is likely to be subjected to further analysis, the extracts should be purified first; excess silver ions and 2,7-dichlorofluorescein can be removed by passing the extract through small silica columns or by washing with bicarbonate, ammonia or sodium chloride solutions [42].
Finally, a very different TLC technique should be mentioned. The concept is based on using quartz rods (Chromarods™), instead of glass plates, coated with a fused layer of a sorbent such as silica gel or alumina; a flame ionization detector (FID) in the Iatroscan™ analyser is utilized to detect and quantify the separated species. Sample application and development are similar to conventional TLC, but one advantage of TLC-FID is that the rods can be reused. TLC-FID has been adapted to many analytical problems, and has been applied in the silver ion mode to lipids. Since there is no difference in the principle of the separation mechanism and few applications to real samples (as opposed to model mixtures) have been described, TLC-FID is not discussed further here. A detailed description of the technique has been published [1], and a review of applications to lipid analysis is available [2].
2. Separation of Fatty Acid Derivatives
From the first introduction of silver ion chromatography for lipid analysis in 1962, it has been evident that fatty acids could be resolved on the basis of the number, the configuration and to some extent the position of the double bonds [131,196]. Since then, silver ion TLC has been utilized intensively to provide invaluable information on the fatty acid structure and content of numerous natural and modified oils and fats and of lipid samples of terrestrial and marine origin. Usually, the fatty acids are subjected to silver ion TLC in the form of methyl ester derivatives, but resolution of free acids [13] and of ethyl and chloroethyl esters [24] is also possible.
Most of the separations reported so far have been on the basis of the number of non-conjugated (methylene-interrupted) double bonds. Resolution of fatty acids with zero to three double bonds is now considered as routine [86,89,93,96,98,128,131]. Mixtures of hexane-diethyl ether or benzene-hexane in different proportions (often between 90:10 and 80:20 by volume) are frequently used as developing solvent. All approaches for impregnating the adsorbent layer have been explored with the percentage of silver nitrate varying between the wide limits of 0.5 [146] to 30% [131].
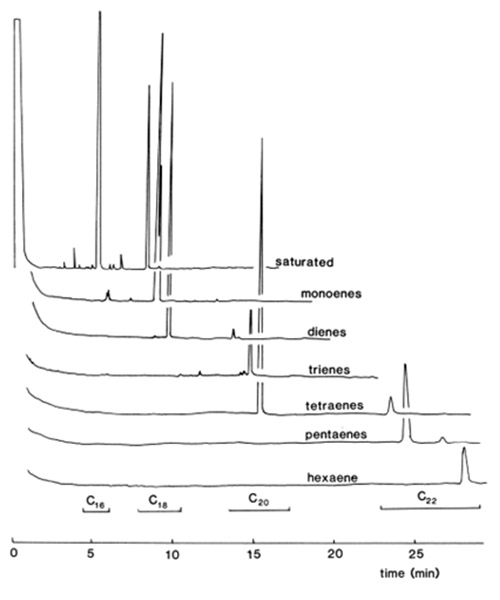
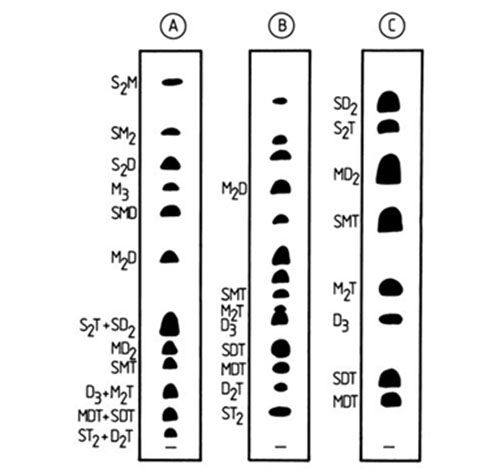
As the resolution of a mixture with zero to six double bonds is more difficult, this is usually attempted in two stages. For example [18], a plate was first developed with a polar solvent mixture, e.g. chloroform-methanol-water (80:20:2 by volume), when fatty acid methyl esters (FAME) with three to six double bonds were resolved; saturated, monoenoic and dienoic components, which formed one or two zones, could either be scraped from the plate and resolved on their own [18], or the separation could be continued on the same plate with a less-polar mobile phase (see above) [65,86,93,98,169,181]. An example is shown in Figure 3. Similar separations were accomplished by a double development with hexane-diethyl ether-acetic acid (94:4:2 by volume) on a plate containing 9% silver nitrate [65]. Alternatively, the method of Inomata et al. [98] appears to have been well accepted by lipid analysts; FAME were resolved on pre-coated silica plates, impregnated by spraying with 40% aqueous silver nitrate, and given a single development with benzene-ethyl acetate (9:1, v/v). Among many examples, these general methods have been applied in the analysis of fatty acids in marine organisms [90,91] and in brain [154].
Figure 3. GLC recorder traces of fatty acid methyl esters separated into saturated, mono-, di-, tri-, tetra-, penta- and hexaenoic fractions by TLC on silica gel G impregnated with 10% (w/w) silver nitrate [42]. Plate A, mobile phase of hexane-diethyl ether (90:10, v/v); plate B, mobile phase of hexane-diethyl ether (40:60, v/v). (Reproduced by kind permission of the author and of Pergamon Press, and redrawn from the original).
Among the more important achievements of silver ion TLC has been the separation of geometrical isomers. It is well established that trans isomers migrate ahead of the corresponding cis compounds [77], and very good resolution was achieved when geometrical isomers derived by stereo-isomerization of linoleic (cis-9,cis-12-18:2) [112,152,196] and linolenic (cis-9,cis-12,cis-15-18:3) [196] acids were examined. The migration order was tt > ct > cc and ttt > ctt > cct > ccc, respectively. However, the complex mixture of all the possible isomers has not been resolved on a single plate, and the formation of a number of mixed zones should be expected [196]. For example, the migration order of C18 FAME with zero to two double bonds was - saturated > trans-monoenes > cis-monoenes plus trans,trans-dienes > trans,cis- or cis,trans-dienes > cis,cis-dienes [97]. The critical pair, cis-monoenes and trans,trans-dienes, has been resolved at ambient temperature on plates impregnated with silver benzenesulfamate and developed with hexane-pentane-diethyl ether-acetic acid (100:30:6:3 by volume) [97].
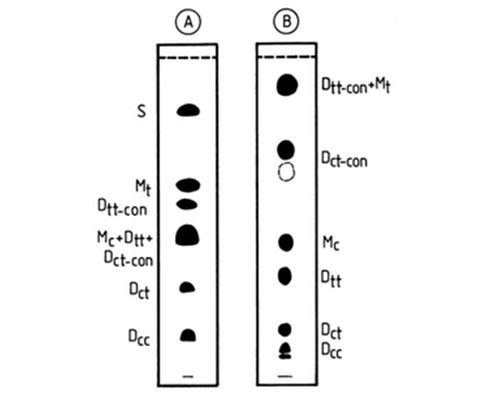
With the more complex mixture of saturated, trans-monoene, trans,trans-conjugated diene, cis,cis-conjugated diene plus cis,trans-conjugated diene, cis-monoene, trans,trans-diene, cis,trans-diene and cis,cis-diene (migrating in this order), complete resolution was achieved on two different plates [38] (Fig. 4). On the first plate, impregnated with 0.5% silver nitrate and developed in sequence with petroleum ether-acetone (100:2, v/v; 2 mL) and petroleum ether-acetone (100:0.7, v/v; 3 mL), only the cis,trans-conjugated diene, cis-monoene and trans,trans-diene remaining as a mixed zone. To resolve this, the whole sample was applied to a second plate, impregnated with 1% silver nitrate, and this was developed with either chloroform (8 mL; with the stabilizing ethanol removed) or 0.4% (by volume) methanol in chloroform (5 mL). The separations were performed in the open cylindrical tanks discussed above. Some conjugated octadecatrienoates were resolved also (as distinct fractions) at −20°C on a plate impregnated with 30% silver nitrate and developed with toluene [69].
While these separations illustrate the potential of the technique, complex mixtures of FAME that contain geometrical isomers of dienoic and trienoic fatty acids cannot be fully resolved by applying silver ion TLC only. On the other hand, silver ion TLC may be the easiest and cheapest way to determine the trans-monoene content of dietary fats [31,56,186]. Usually, the trans-monoenes are completely separated from the saturated and the cis-monoenoic fatty acids under the chromatographic conditions established for resolution of saturated, monoenoic and dienoic components [38,96,131]. A clear separation, for example, can be achieved on self-prepared plates (19 cm) by developing with petroleum ether-acetone (100:4, v/v; 3.0-3.5 mL) in the open cylindrical tanks described above. The separation worsens, however, when the sample contains a variety of fatty acids differing in chain length and in the positions of the double bonds [177].
Figure 4. Separation patterns of standard mixture of isomeric fatty acid methyl esters, resolved by silica gel G silver ion TLC in open cylindrical tanks [38]. Plate A, the layer was impregnated with 0.5% methanolic silver nitrate and the plate was developed with 2 mL petroleum ether-acetone (100:2, v/v) followed by 3 mL petroleum ether-acetone (100:0.7, v/v). Plate B, the layer was impregnated with 1.0% methanolic silver nitrate and the plate was developed with 8 mL chloroform. The spots were detected by treating the plate in sequence with bromine and sulfuryl chloride vapours, followed by heating at 180-200°C. S, M and D denote saturated, monoenoic and dienoic fatty acid methyl esters; c - cis, t - trans and con - conjugated double bonds. (Reproduced by kind permission of the Journal of Planar Chromatography - Modern TLC).
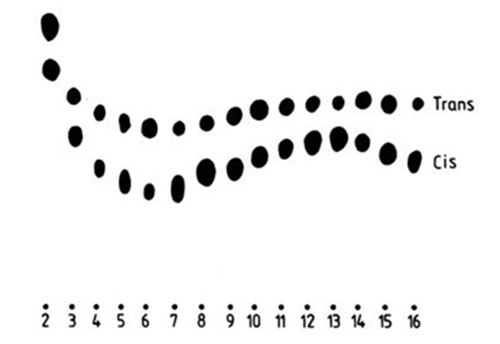
To separate different positional isomers of unsaturated fatty acids has always been a challenge for silver ion chromatography. The elution behaviour on silver ion TLC of all the cis- and trans-octadecenoates [77], all the methylene-interrupted cis-octadecadienoates [41], many cis- and trans-dimethylene-interrupted octadecadienoates [119], all the octadecynoates [21] and many octadecadiynoates [119] have been studied. When applied in sequence on a single plate, each series migrated in the form of a sinusoidal curve (Fig. 5).
Figure 5. Silver ion TLC of methyl cis- and trans-octadecenoates [77]. The plate was impregnated with 15% (w/w) silver nitrate and developed with dibutyl ether-hexane (40:60, v/v); spots were visualized by heating with a glass-blower's torch. The numbers on the bottom refer to the position of the double bond in the fatty acid molecule. (Reproduced by kind permission of the authors and of Chemistry and Physics of Lipids, and redrawn from the original).
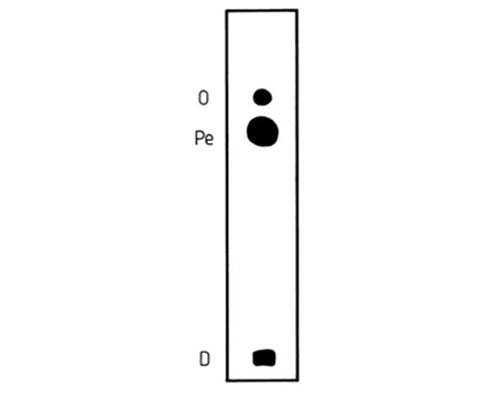
There were minima at the 6c-, 6a-, 6c,9c- and 6a,10a-isomers and maxima at the 13c-, 16a- and 10a,14a-isomers in the appropriate series. (The series of methylene-interrupted octadecadienoates did not have a distinct maximum, and that of the dimethylene-interrupted trans-octadecadienoates did not have a distinct minimum). In each instance, the first member of the series, i.e. with a double or triple bond in position 2, migrated well ahead of all other members. Indeed, trans-2-octadecenoate migrated ahead of stearate [77]. Unfortunately, the differences in Rf values were often too small to be of practical value, and resolution of simple mixtures only of positional isomers of fatty acids from natural sources has been reported so far. However, these included methyl petroselinate (cis-6-18:1), oleate (cis-9-18:1) and vaccenate (cis-11-18:1) [28,135,146], which often occur together in tissues and are not always easy to separate otherwise. All the published separations have confirmed a requirement for low temperatures, i.e. −20°C, to successfully effect the required resolution [28,135,146]. In contrast, the high silver nitrate content (30%) described by Morris et al. [135] does not appear to be critical. Breuer et al. [28,29] reported excellent resolution of cis-9-18:1 and cis-6-18:1 FAME on pre-coated alumina plates, impregnated by immersing for 30 seconds in 10% silver nitrate, and given a single development with toluene. Similarly, a separation of this isomeric pair was achieved on a home-made silica gel plate, impregnated by dipping in 1% methanolic silver nitrate, and given a continuous development with petroleum ether-diethyl ether (100:5, v/v; 5 mL) [146] (Fig. 6).
Figure 6. Separation of cis-6-18:1 (Pe) and cis-9-18:1 (O) fatty acid methyl esters from Pimpinella anisum seed oil by silver ion TLC [146]. The silica gel layer was impregnated with 1% silver nitrate and the plate was developed in an open cylindrical tank with 5 mL petroleum ether-ethyl ether (100:5, v/v) at −20°C. The spots were detected by treating the plate in sequence with bromine and sulfuryl chloride vapours, followed by heating at 180-200°C.
Very little is known about the behaviour of the positional and geometrical isomers of longer-chain fatty acids on silver ion TLC plates. Resolutions of a few isomeric cis- and trans-eicosadienoic [129], docosamonoenoic [28,129,176] and docosadienoic [176] acid methyl esters have been reported so far. Again, trans- migrated ahead of cis-isomers, but generally longer-chain fatty acids were less strongly held on the plate. For example, a mixture of stearic, brassidic (trans-13-22:1), elaidic (trans-9-18:1), erucic (cis-13-22:1), vaccenic, oleic, petroselinic, linoleic and linolenic acids migrated and were separated in this order on a single alumina plate impregnated with 2% silver nitrate and with toluene-hexane (60:40, v/v) as the mobile phase at -20°C [28]. With more complex mixtures of different isomeric fatty acids with a greater variety of chain lengths, the formation of numerous mixed zones might be expected [129,176]. Therefore, from a practical point of view, it is more reasonable to combine silver ion TLC with another separation technique. For example, fatty acid methyl esters were resolved first according to chain length by preparative GC and the individual fractions then subjected to silver ion TLC [177,178]. A more effective approach is, however, to fractionate the sample first by silver ion TLC according to degree of unsaturation and then to apply capillary GC [177] or reversed-phase HPLC [18].
A relatively new problem is the separation of very-long-chain (C24 to C36) polyunsaturated fatty acids [16,17,154]. For example, a sample of bovine retina fatty acids was resolved on silica gel containing 20% silver nitrate (incorporated into the layer), with a mobile phase of chloroform-methanol (95:5, v/v) [17]. In this instance, three fractions, i.e. tetra-, penta- and hexaenoic fatty acids, were obtained and each was then examined further by GC.
Substituted fatty acids can also be effectively separated on the basis of degree and type of the unsaturation. A wide range of unsaturated epoxy, halohydroxy, hydroxy and dihydroxy fatty acids have been separated by silver ion TLC, and the results were reviewed by Morris and Nichols [134]. Similarly, silver ion TLC was applied in the analysis of cyclopentenyl [126,127] and furanoid fatty acids [80]. Silver ion TLC was found to be of special value in such elegant separations as in the determination of the absolute optical configuration of (+)-threo-12,13- and (-)-threo-12,13-dihydroxyoleic acid [133]. The two isomeric threo-12(13),13(12)-hydroxy,tosyloxy derivatives, derived from methyl vernolate and from methyl dihydroxyoleate, were separated by preparative silver ion TLC and given a double development with petroleum ether-diethyl ether (1:1, v/v). The 13-hydroxy,12-tosyloxy isomer migrated more rapidly, and this was ascribed to the diminished ability of the double bond to complex with silver ions because of steric hindrance by the bulky tosylate group or to delocalization of the pi-electrons of the double bond by this group or to both.
Thus, silver ion TLC offers an effective means of fractionation of complex fatty acid mixtures into distinct fractions differing in the number, and in some cases the type and position of double bonds. When such fractions are subjected to GC or, better, to GC/MS, it is possible to assign the number, position and configuration of double bonds with much greater confidence. Silver ion TLC serves also as an enrichment procedure for minor components and allows more accurate estimation of their content and identity.
3. Separation of Molecular Species of Triacylglycerols
In 1962, Barret, Dallas and Padley [19] described the first application of silver ion TLC to the analysis of triacylglycerols, and since then this approach has been intensively explored to elucidate the structures of natural fats and oils.
Separations are based mainly on the overall degree of unsaturation of the triacylglycerol molecule; but of two species with an equal number of double bonds, that in which all of the double bonds are concentrated into one fatty acyl moiety is held more firmly. Thus, a triacylglycerol with two saturated and one linoleoyl moiety is more strongly retained than one with one saturated and two monoenoic acyl groups. A mixture which contains the common range of C16 to C18 fatty acids with up to three double bonds can be resolved into 20 species. Components migrate in the order:
SSS > SSM > SMM > SSD > MMM > SMD > MMD > SDD > SST > MDD > SMT > MMT > DDD > SDT > MDT > DDT > STT > MTT > DTT > TTT
where S, M, D and T denote saturated, mono-, di- and trienoic fatty acyl moieties respectively (but do not indicate the position in the molecule) [78,165]. Depending on the nature of the mobile phase, some changes in the order of triacylglycerols which contain trienoic fatty acids may occur, and care must be taken with identifications. Generally, separation of triacylglycerols with more than six double bonds is not easy, although distinct resolutions of all species up to trilinolenin have been achieved by skilled analysts [189,190]. The results of attempted resolution of more highly unsaturated species, such as those of fish oils [27], have not been impressive, and much remains to be done to improve the technique if it is to be used for such samples.
As in the previous section, separations of triacylglycerol have been performed on home-made or pre-coated plates impregnated with silver nitrate, and again the optimum concentration of silver ions remains an open question. Successful fractionations have been described with 10 to 20% silver nitrate in the layer. However, similar resolutions were achieved after impregnation by immersion or spraying with silver nitrate solutions with the same concentrations, which meant that the silver nitrate contents of the layers were much lower. Indeed, excellent separations were reported with plates impregnated by immersion in only 0.5% methanolic silver nitrate [35,37,189,190].
Mobile phases have been composed of two-component systems mainly. Chloroform-methanol mixtures seem to be the best choice, although a number of others have been successfully employed, for example, benzene-diethyl ether [78,201], petroleum ether-acetone [37], toluene-hexane [10], chloroform-acetic acid [19], tetrachloromethane-acetic acid [29], benzene-cyclohexane or benzene-cyclooctane [30], toluene-chloroform [58], benzene-methanol [159], and others. Many of these solvents are now considered too hazardous for routine use. The proper choice of solvents and especially the proportions depend on various considerations, but mainly on the nature of the mixture to be analysed. However, all the factors described above (Section C.1), i.e. temperature, humidity and the activity of the sorbent, may affect the separation and require an adjustment to the proportions of the solvents in the mobile phase. Despite the theoretical achievements in understanding the properties of the more widely used solvents (see for example [44,166,184]), it seems that lipid analysts choose mobile phases mainly on an empirical basis or by modifying previously reported compositions.
The author recommends that triacylglycerols with up to six double bonds be separated on a single 20 cm long plate with 1% methanol in chloroform as the mobile phase. To resolve the remaining highly unsaturated fractions, an aliquot of same sample should be applied to another plate for development with a higher proportion of the polar component in the mobile phase, e.g. 5% methanol in chloroform. For a precise separation and determination of the triacylglycerols in seed oils of citrus fruits, three different plates and five different mobile phases were used [189,190]. This was not necessarily time-consuming, since all of the plates could be developed simultaneously in different tanks (Fig. 7). Conventional separations are carried out in closed rectangular tanks, sometimes lined with absorbent paper to saturate the atmosphere with solvent vapour. The volume of the mobile phase, which is very important in the author’s opinion, is never specified and only the duration of the development is occasionally mentioned. It seems that only the author and colleagues use continuous development in open tanks [11,37,100,145,146,189,190].
Figure 7. Separation of triacylglycerols from orange seed oil by silver ion TLC [189]. The silica gel G layer was impregnated with 0.5% methanolic silver nitrate and plates were developed in open cylindrical tanks. Plate A, mobile phase of 8 mL petroleum ether-acetone (100:6, v/v); plate B, mobile phase of 5 mL petroleum ether-acetone (100:8, v/v) followed by 8 mL petroleum ether-acetone (100:5, v/v); plate C, mobile phase of 4 mL petroleum ether-acetone (100:7, v/v), followed by 15 mL petroleum-ether acetone (100:4, v/v). Spots were detected by treating the plates in sequence with bromine and sulfuryl chloride vapours followed by heating at 180-200°C. The total sample was applied on each plate. S, M and D denote saturated, monoenoic and dienoic fatty acyl residues. (Reproduced by kind permission of the authors and of La Rivista Italiana delle Sostanze Grasse, and redrawn from the original).
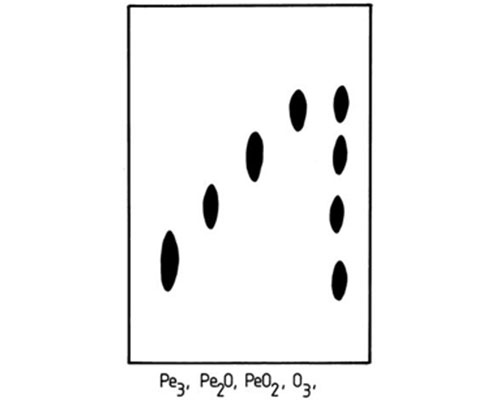
From the first attempts to resolve triacylglycerols, it was evident that silver ion TLC has the capacity to distinguish between species which contain isomeric fatty acids. Thus, the migration order of triacylglycerols which contained saturated, oleic (O) and elaidic (E) acids was SSS > SSE > SEE > SSO > EEE > SOO > OOO [10,196,201]. With triacylglycerols containing oleic (O) and petroselinic (Pe) acids, OOO, PeOO, PePeO and PePePe were resolved in this order on a plate with 23% silver nitrate in the layer and given a triple development with toluene-diethyl ether (75:25, v/v) at −23°C [201] (Fig. 8). These appear to be the only examples of separation by silver ion TLC of triacylglycerols differing in the geometry and position of double bonds in the fatty acyl moieties, and the analytes were simply reference mixtures. The picture would be much more complicated with samples of natural origin, where other acids would be present. Therefore, with a few exceptions [10,58], such separations may have little practical value.
Figure 8. Separation of triacylglycerols that contain positional isomers of unsaturated fatty acids by silver ion TLC [201]. The silica gel G layer was impregnated with 23% (w/w) silver nitrate, and the plate was developed three times with toluene-diethyl ether (75:25, v/v) at −20°C. The spots were visualized by spraying with 2,7-dichlorofluorescein solution. Pe and O denote petroselinic and oleic fatty acyl residues, respectively. (Reproduced by kind permission of the authors and of Fat Science and Technology, and redrawn from the original).
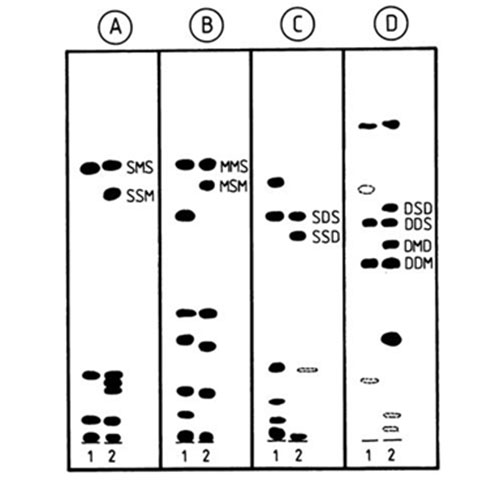
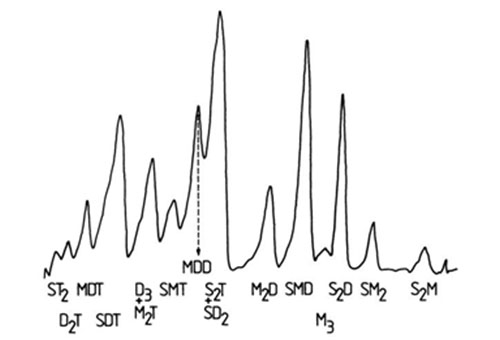
One of the more valuable achievements of silver ion chromatography has been the unique ability to resolve positional isomers of triacylglycerols, i.e. in which the position of a given fatty acid moiety on the glycerol backbone varies, including the pairs SSM-SMS, MMS-MSM, SSD-SDS, MMD-MDM, DDS-DSD, DDM-DMD (positions sn-1 and sn-3 are not differentiated), as well as the three positional SMD isomers. The pairs SSM-SMS and SSD-SDS are resolved with relative ease at ambient temperature with mobile phases such as chloroform-methanol-acetic acid (60:40:0.5 by volume) [19,20], toluene-chloroform (1:1, v/v) [58], toluene-diethyl ether (96:4, v/v) [72]. The “symmetrical” isomers SMS and SDS migrate ahead, probably because the unsaturated fatty acid complexes less strongly with silver ions when located in the middle position, due to steric or electronic factors or both. The pair MMS-MSM has also been resolved [72], and here the MMS isomer moved ahead. All of the isomeric pairs mentioned above, except the MMD-MDM and all the SMD isomers, have been separated by a continuous development in open tanks with chloroform-methanol mixtures [145] (Fig. 9). The proportions of silver nitrate in the adsorbent layer and of methanol in chloroform in the mobile phase were gradually increased according to the degree of unsaturation of the species to be resolved. The pairs DDS-DSD and DDM-DMD were separated on a single 20 cm long plate, impregnated by immersion with 2% methanolic silver nitrate and given consecutive developments with 2.5% methanol in chloroform (6 mL) and 1.5% methanol in chloroform (15 mL) [145]. With these pairs, the DSD and DMD isomers, respectively, moved ahead.
Figure 9. Separation of positional isomers of triacylglycerols by silver ion TLC [145]. Plates A, B and C were impregnated with 1% methanolic silver nitrate, and plate D was impregnated with 2% methanolic silver nitrate. The plates were developed in open cylindrical tanks. Plate A, 12 mL of 0.5% (v/v) methanol in chloroform; plate B, 14 mL of 1.0% (v/v) methanol in chloroform; plate C, 16 mL 1.0% (v/v) methanol in chloroform; plate D, 6 mL of 2.5% (v/v) methanol in chloroform followed by 15 mL 1.5% (v/v) methanol in chloroform. S, M and D denote saturated, monoenoic and dienoic fatty acyl residues, respectively. Sample 1 was grapefruit seed oil; sample 2 was randomized lard (plates A and B), randomized mixture of sunflower oil and tristearin (plate C), and randomized sunflower oil (plate D).
The ability to separate positional isomers of triacylglycerols is of real practical value. For example, it is possible to identify species with specific fatty acids in position sn-2 of the molecule without having to undertake enzymatic hydrolysis [145]. Moreover, the SSM, MSM and SSD species are present in negligible amounts in natural vegetable oils and fats, but are normal constituents of interesterified fatty products. Therefore, silver ion TLC offers a simple way to detect adulteration in dietary fats. Some examples have been reported, as in the analysis of cocoa butter [58] and olive oil [72], but the above procedures can also be applied to more unsaturated oils.
Silver ion TLC has had and indeed continues to have an important role in determining the triacylglycerol structure of natural and modified fats and oils. Used alone or more often for preliminary preparative-scale simplification of complex mixtures, silver ion TLC provided invaluable information on the composition of such oils and fats as lard [20,26,37], cocoa butter [20,37], shea butter [20], milk fat [122], olive oil [37,201], sunflower oil [37,79,201], palm kernel oil [201], cod liver oil [27], citrus seed oils [189,190], peanut oil [23,79,201], cottonseed oil [23,75,201], evening primrose oil [159], tallow [37], corn oil [26], maize oil [61,79], linseed oil [78], soybean oil [78,149], safflower oil [79], tobacco oil [79], mowrah butter [79], stillingia oil [78], wild rose oil [78] and ucuhuba kernel oil [57]. Similarly, silver ion TLC has been applied successfully to study processes which lead to changes in triacylglycerol composition, such as maturation of seeds [165] and effects produced by heating [209] or during the process of interesterification [39]. This list is by no means comprehensive, and Litchfield’s review [121] provides many more examples.
4. Separations of Molecular Species of Complex Lipids
Silver ion TLC has been applied less frequently to the analysis of complex lipids but is of value for phospholipids especially. It is usually employed for detailed analyses of a given lipid class, which as before is resolved into simpler species differing in degree of unsaturation. There are two general approaches to the problem. One is to separate intact phospholipids, sometimes after masking polar moieties by suitable derivatization; this may then permit biochemical studies of the rate of turnover of every part of the molecule when radioactive labels are used. The second strategy consists in hydrolysing the phospholipid to remove all or part of the polar head-group, thereby greatly simplifying the practical problems and increasing the scope for analysis by complementary means.
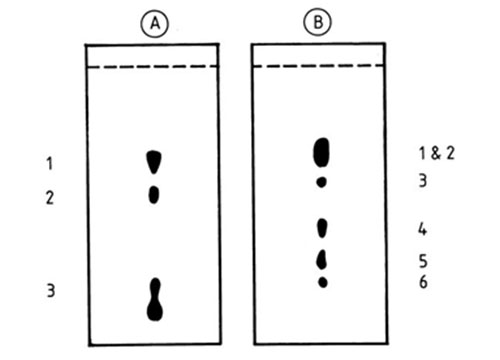
With technical improvements in reversed-phase HPLC methodology, silver ion TLC of intact phospholipids has fallen out of favour in recent years because of the technical difficulties. The early literature has been reviewed by Christie [42], and a few key papers only are described here. Silica gel H (i.e. without binder) impregnated with silver nitrate is the sorbent of choice for intact phospholipids, while silica gel G is preferred for derivatized lipids. A procedure described by Arvidson [14,15] is the only one to have been applied successfully to intact phosphatidylcholines to resolve molecular species with zero to six double bonds (Fig. 10). Silica gel H containing silver nitrate (25% w/v) was employed and plates were activated under stringent conditions, i.e. at 175°C for 5 hours or 180°C for 24 hours. With the less active plates, all but the saturated and monoenoic species could be resolved with a mobile phase of chloroform-methanol-water (60:35:4 by volume); the problem pair was resolved on the more active plates. Native phosphatidylethanolamine was resolved under the same conditions [15], but many analysts have preferred to convert to nonpolar derivatives, while retaining both the phosphorus and ethanolamine moieties [162,185,207]. Related procedures have been employed for phosphatidylserine [167,207] and phosphatidylinositol [92,123]). For example with the latter [107,123], acetylation and methylation were utilized to mask the free hydroxyl and phosphorus groups, respectively, and this permitted resolution of molecular species on silver nitrate-impregnated layers with chloroform-acetone mixtures as the mobile phase. The use of isotopically labelled acetate for derivatization in this instance enabled quantification of fractions by liquid scintillation counting.
Figure 10. Fractionation of native phosphatidylcholines by silver ion TLC [14]. The silica gel H layers were impregnated with silver nitrate (10:3, w/w). A, plate was activated at 180°C for 24 hr; B, plate was activated at 175°C for 24 hr; the mobile phase was chloroform-methanol-water (65:25:4, v/v). The numbers alongside the plates refer to the number of double bonds in each species. (Reproduced by kind permission of the author and of the Journal of Lipid Research, and redrawn from the original).
Methods of the same kind have also been employed for ceramides derived from cerebrosides [81], sulfoquinovosyl diacylglycerols [141] and mono- and digalactosyldiacylglycerols [168] amongst others. Phospholipase D has been used to convert phospholipids to phosphatidic acid, which was rendered nonpolar by reaction with diazomethane to form dimethylphosphatidate. With such derivatives, it is still possible to study the mechanism of turnover of the phosphorus moiety. They can be separated by silver ion TLC into fractions with zero to six double bonds under relatively mild conditions (e.g. chloroform-methanol-water (90:10:1 by volume) as mobile phase) [162]; reversed-phase TLC is also possible but high-temperature GC is not. The method has been applied to a variety of different phospholipids [107] and lysophospholipids [108]. In an interesting departure from conventional practice, Kennerley [109] hydrolysed phospholipids with phospholipase C to diacylglycerols, which were converted to isotopically labelled phosphatidic acid by reaction with [32P]-ATP and diacylglycerol kinase; the resulting derivatives were separated both by silver ion and reversed-phase TLC, and they were detected and quantified with relative ease via the label. The procedure was subsequently applied to study the metabolism of diacylglycerols per se in mast cells [110].
On the other hand, the most popular approach to the analysis of molecular species of phospholipids has consisted in hydrolysis with phospholipase C to diacylglycerols and conversion to nonpolar derivatives such as acetates or silyl ether derivatives. Kuksis and Myher [115] recommended the use of a specific phospholipase C from Bacillus cereus (Type XIII, Sigma Chemical Co.) for the purpose, since it is active towards most phospholipids including phosphatidylserine and phosphatidylinositol. Alternatively, it is possible to prepare diacylglycerol derivatives by direct acetolysis of phospholipids with acetic acid and acetic anhydride (at 140°C overnight), although this will lead to degradation of plasmalogens [40,160]. While the latter procedure also causes some isomerization of 1,2-diacylglycerols to the 1,3-form, this need not be troublesome in analyses of molecular species. The strategy of separating molecular species of phospholipids via diacylglycerol derivatives has the advantage that silver ion TLC is accomplished under mild conditions, and most complementary analytical techniques, including reversed-phase TLC and HPLC and high-temperature GC can be employed.
For example, diacylglycerol acetates with zero to six double bonds have been resolved in a single run with silica gel G impregnated with silver nitrate (10% w/w) and with chloroform-methanol (99:1, v/v) as mobile phase [42]. Such compounds generally migrate in the order:
SS > SM > MM > SD > MD > DD > ST > MT > STe > MTe > DTe > SP > SH
Renkonen [161] separated species with up to twelve double bonds with a mobile phase of chloroform-methanol-water (65:25:4 by volume). Others have resolved tert-butyldimethylsilyl ether [138,140] and benzoyl ester [25] derivatives of diacylglycerols by analogous procedures. As an example, a remarkable separation of molecular species of phosphatidylethanolamines, isolated from Escherichia coli grown with elaidate, has been reported [147]. The derived diacylglycerol acetates were applied to a plate impregnated with 10% silver nitrate in acetonitrile (the plate was immersed in this solution for 30 minutes and then activated at 148°C for one hour). After development with benzene-chloroform-methanol (98:2:0.1 by volume), the mixture was resolved into seven fractions differing not only according to the number and configuration of the double bonds but also to the chain length of the fatty acid moieties, i.e. 16:0/t-18:1 16:0/t-16:1 t-18-:1/t-18:1 t-18:1/t-16:1 > c-18:1/t-18:1 c-16:1/t-18:1. In addition, such procedures have been used with cardiolipin [105,208], and for alk-1-enylacyl, alkylacyl and diacyl subclasses of phospholipids [25].
In the analysis of complex lipids, silver ion TLC has been applied mainly in a preparative mode. Fractions are visualized in the conventional way under UV light with a fluorescent indicator, the adsorbent bands are carefully removed and the material is extracted from the sorbent. Extraction by the procedure of Folch et al. [71] is often recommended for underivatized phospholipids, and extracts should be washed with sodium chloride solution in order to remove the excess silver ions before components are submitted to further analysis [14,15,42] (see also Section C.1 above).
5. Quantification
Silver ion TLC has been represented so far as an inexpensive, easy-to-perform and efficient technique to resolve complex mixtures of lipids into simpler fractions. It must also provide quantitative information. The most widely applied procedure is indirect quantification by extracting the fractions from the adsorbent layer in the presence of an internal standard, transmethylating and subjecting the methyl esters of the fatty acids to GC analysis; information is obtained simultaneously on the composition of the fractions and their absolute amounts. In practice, the sample is resolved on a preparative plate, each distinct zone is carefully scraped off, a standard solution of the internal standard (usually an odd-chain methyl ester) is added and the material is extracted with a suitable polar solvent, such as diethyl ether or a chloroform-methanol mixture. More complicated extraction procedures are sometimes needed for polar complex lipids, and they can be found in the original papers (see Section C.3 above) or in the book by Christie [42]. Extracts are purified as already described (Section C.1 above). The further steps depend on the nature of the lipids, and fatty acid methyl esters can be subjected directly to GC, for example. On the other hand, triacylglycerols and other lipids are usually transmethylated with the internal standard before analysis by GC. Methods for transmethylation were reviewed by Christie [47] (and a further more recent review is available on this website here). Alternatively, diacylglycerols derivatives and triacylglycerols have been subjected immediately to analysis by high temperature GC in the presence of an internal standard such as a short-chain triacylglycerol, e.g. tridecanoin [47].
As an alternative, a densitometric technique has been developed to quantify the separated species directly on the silver ion TLC plate. The basis of the method is the difference in optical response between the blank part of the plate and regions where the analytes are present. Nowadays, excellent instrumentation is available [191], and the problems that arise are rarely a function of the densitometer and depend mainly on the properties of the chromatogram. A clean background and well-resolved, distinct and evenly stained zones are required. In most instances, the staining procedure is the critical step.
Lipids do not possess any chromogenic groups and are usually visualized for direct quantification by charring and carbonization. Although charring is a sensitive detection procedure, it is not easy to control and all steps, including treatment of the plate with the charring reagent, and the temperature and duration of heating, must be standardized as far as is possible in order to obtain reproducible results. The most common procedure is to spray the plate with a 50 to 70% aqueous methanolic or ethanolic solution of sulfuric acid, but other oxidizing reagents, such as 70% sulfuric acid saturated with potassium dichromate [98] or chlorosulfonic acid-glacial acetic acid [82], have given good results. However, spray reagents of this type are extremely corrosive, and spraying is an inherently inconvenient procedure, so alternative approaches have been proposed. For example, the developed plate has been treated with vapours of sulfuryl chloride [37], which saturate the silica layer and decompose to sulfuric acid rapidly when the plate is heated at 180 to 200°C. Similar results have been achieved by incorporating silver sulfamate instead of silver nitrate into the layer [96], as sulfamate also decomposes to produce sulfuric acid when heated (but insufficient to carbonize saturated species completely). In addition, phosphotungstic acid has been proposed as a charring reagent for incorporation into the layer [13]. On heating at 220 to 250°C, the lipids were oxidized and silver ions were reduced when intense red zones, which were independent of degree of unsaturation, appeared on a pale background. In general, the incorporation of charring reagents into the sorbent layer should be very carefully controlled, since this may seriously change its properties, especially the silver ion complexation, and thence affect the resolution in an unpredictable way.
An important requirement for quantitative purposes is that the charring reagent should react in an equal manner with all components, and in particular, staining should not be influenced by the different degree of unsaturation of the separated species. If this is not possible, correction factors should be introduced with the densitometric response of one of the components taken as a standard.
Plates with carbonized zones are scanned in the densitometer at 400 to 450 nm. When other staining procedures are used that produce visible- or UV-absorbing zones, a working wavelength should be chosen that lies in the region of maximum absorption. Zig-zag (or flying spot) scanning is greatly superior to linear scanning and should be preferred. Densitometers must be coupled to a recorder and ideally to an integrator, so that the instrument not only monitors the chromatogram but quantifies the peaks in the most accurate, reproducible and convenient way.
As an example, fatty acids with zero to four double bonds were determined densitometrically after spraying the plate with 70% sulfuric acid saturated with potassium dichromate and heating at 120°C for one hour. Linear calibration graphs were obtained for each component in the range of 35 to 150 nmol. The molar response of methyl oleate was found to be less than that of methyl stearate, while those of unsaturated fatty acids increased with increasing chain length and number of double bonds [98]. On the other hand, the effect of differences in unsaturation could be minimized by treating the developed plate with bromine vapour prior to charring [38] (Fig. 11). It has been claimed that silver ion TLC-densitometry is the easiest and cheapest way to determine the trans-monoene content of dietary fats [70].
Figure 11. Densitograms of isomeric fatty acid methyl esters, separated by silver ion TLC [38]. For the chromatographic conditions see Figure 4 (above), plates A and B. Spots were visualized by treating the plate in sequence with bromine and sulfuryl chloride vapours, followed by heating at 180-200°C. S, M and D denote saturated, monoenoic and dienoic fatty acid methyl esters respectively; c, cis; t, trans; con, conjugated double bonds. (Reproduced by kind permission of the Journal of Planar Chromatography - Modern TLC).
The first application of densitometry for quantification of triacylglycerols was described in a classic paper by Barret, Dallas and Padley [20]. The zones were visualized by spraying with 50% aqueous orthophosphoric acid and heating at 340°C. As the response was found to depend strongly on the degree of unsaturation of the species, correction coefficients were introduced, but the calibration graphs were linear in the range 0.5 to 6 µg. This procedure was improved significantly by Chobanov et al. [37]. First, the silver nitrate content of the adsorbent was lowered from 12.5% by using an immersion procedure with 0.5% methanolic silver nitrate (Fig. 12). This made charring easier and simplified the densitometric determination. Second, the need for correction factors was eliminated by treating the developed plates initially with bromine vapour and only then with the vapour of sulfuryl chloride. Bromine was assumed to react with double bonds, producing the bromo derivatives, so that the quantitative results did not depend on unsaturation and the relative peak areas represented accurately the real composition of the mixture analysed. Recently, this procedure was carefully re-examined using a Shimadzu CS-930 zig-zag scanner and model triacylglycerols with zero, three and six double bonds [143]. The linear range was found to be 1.0 to 10 µg per spot, determined with good reproducibility and accuracy. Hence, the densitometric determination of triacylglycerols [37] is in no way inferior to any other approach for quantification per se.
Figure 12. Densitogram of lemon seed oil triacylglycerols separated by silver ion TLC [190]. For the chromatographic conditions, see Figure 7 above, plate A. The spots were visualized as in Figure 11. S, M and D denote saturated, monoenoic and dienoic fatty acyl residues, respectively. (Reproduced by kind permission of the authors and of La Rivista Italiana delle Sostanze Grasse, and redrawn from the original).
However, carbonization by charring is not generally accepted as the ideal quantification procedure, and the search for new approaches to visualize lipids has continued. Hammond [82], for example, incorporated phloxyn into the layer and measured the secondary fluorescence through a cut-off filter at 560 nm. The presence of silver nitrate appeared to quench the background fluorescence severely, while the lipid material either prevented quenching or enhanced the fluorescence. Among the disadvantages, the method required rigorous standardization of each TLC plate and relied on a well-trained technician. It therefore seems doubtful that this technique will replace simple charring. Lipid analysts have often been skeptical about the possibilities for quantification offered by densitometry, their opinions generally being formed by earlier experience when the instrumentation was quite primitive. Now, the situation is changed greatly and laboratories whose staff is well trained in silver ion TLC methods can successfully apply densitometry. It can be claimed that the overall procedure is less costly and more suitable for routine analysis of large number of samples than any alternative technique.
Abbreviations
FAME, fatty acid methyl esters; FID, flame-ionization detector; GC, gas chromatography; HPLC, high-performance liquid chromatography; MS, mass spectrometry; RP, reversed-phase; SPE, solid-phase extraction; TLC, thin-layer chromatography. With molecular species: S, M, D, T, Te, P and H denote saturated, monoenoic, dienoic, trienoic, tetraenoic, pentaenoic and hexaenoic acyl moieties, respectively.
Nikolova-Damyanova, B. Silver ion chromatography and lipids.In: Advances in Lipid Methodology - One. pp. 181-237 (1992) (Ed. W.W. Christie, Oily Press, Ayr). Published here by kind permission of P.J. Barnes & Associates (The Oily Press), who retain the copyright.