A Guide to the Practice of Analytical Silver Ion-thin-layer chromatography on Pre-coated Silica Gel Plates
The Authors: Boryana Nikolova-Damyanova and Svetlana Momchilova, Institute of Organic Chemistry, Centre of Phytochemistry, Bulgarian Academy of Sciences, Sofia 1113, Bulgaria
1. Introduction
Silver ion-thin-layer chromatography (Ag-TLC) in analytical mode is the method of choice when the task is to check the purity, to identify fatty acid methyl esters (FAME) or triacylglycerol (TAG) component(s), or to get a picture of the composition of a sample. For the purpose, plate dimensions (most often), layer thickness, concentration of methanolic silver nitrate for impregnation, sample load, development chambers and mobile phase volume are scaled down to ensure efficient separation of as many components as possible. In addition to giving credible hints as to identity and purity (comparison with standard mixtures), analytical Ag-TLC presents a true “picture” of the sample composition and, occasionally, allows for in situ quantification of components by scanning densitometry. The preliminary information about the sample supplied by analytical Ag-TLC is essential for the efficient design of preparative Ag-TLC (when required) and of Ag-HPLC or reverse-phase HPLC (RP-HPLC) including the choice of mobile phases, thus adding to the correctness of the final results. Note: Analytical Ag-TLC permits application and simultaneous development of sample and standard under identical chromatographic conditions. This increases the credibility of component purity and identification; only HPLC with mass spectrometric detection can improve upon this.
Various sizes of glass, plastic and aluminium plates pre-coated with silica gel 60 for TLC are commercially available at present (indicated as “common” TLC plates by most of the providers). Of these, we have no experience with plastic-backed and limited experience with glass-backed plates so these will be not discussed further. Note: Purchase plates pre-coated with plain silica gel 60 and keep in mind that introducing silver ions in the layer requires special and longer treatment.
Here we share our practice in the use of aluminium-backed pre-coated commercial plates for analytical Ag-TLC as applied to the analysis of FAME and TAG.
2. Sample preparation
The procedures of extraction of lipids and isolation are described here. The working sample is prepared by dissolving the isolated material in hexane to give a 1.5-2.0% solution.
3. Plate preparation
The binding material in pre-coated silica gel 60 plates is not calcium sulfate (indicated by the G sign in the bulk commercial silica gel for TLC) but an unknown polymer, which ensures, as advertised by one of the providers, “a very adherent and hard surface that will not crack or blister and even allow writing with a pencil on the surface without risk to damage the layer”. The layer is uniform and smooth with a thickness 200-250 μm and particle size of ~10 μm. We use 20 × 20 cm silica gel 60 aluminium-backed plates cut into 4 × 20 and 4 × 10 cm sheets for purity check or identification. Commercial cutters and scissors are available and sheets of a variety of dimensions can be “produced” to suit the aim of the analysis. Further in the text we refer to these sheets as “plates”.
The adhesiveness and hardness of the layer and the unknown binding material do indeed ensure a layer that cannot be mechanically damaged during the chromatographic procedures, but as will be shown below, impregnation, efficiency of the separation and especially detection cause certain problems.
The examples given below deal with aluminium-backed pre-coated silica gel 60 plates. The same, and better, results could be obtained with 5 × 20 cm glass-backed pre-coated plates.
4. Impregnation
Because of the very adherent and hard surface of pre-coated plates, impregnation by simple dipping in or spraying with silver nitrate solution does not provide acceptable results. The plate has to be immersed in the solution (0.5% methanolic solution of AgNO3) for at least 5 min (10 min gives slightly stronger retention and better resolution) as shown in Figure 1A and 1B. The impregnated plates are then left to dry on a rack for two hours in a sheltered place in the laboratory for the methanol to evaporate. Up to 10 min in an oven at 110°C gives the same result.

A

BFigure 1. Impregnation of 4 × 20 cm (A) and 4 × 10 cm (B) sheets of aluminium-backed pre-coated silica gel 60 plates with 0.5% methanolic AgNO3.
5. Sample application
The working sample solution for analytical Ag-TLC depends on the purpose, and for purity checks or identification it is usually 1.5-2% in hexane. An aliquot of 5-10 μL of the solution is applied (automatic or Pasteur pipette), as a spot, at 1.5-2.0 cm above the bottom edge of the plate. The sample amount should not exceed 200 μg (limited by the capacity of the layer) (Fig. 2).

Figure 2. Sample application.
6. Development
The plates are subjected to a single development with 2-4 mL freshly prepared mobile phase. We normally use mobile phases of hexane (or petroleum ether)-acetone in volume proportions of about 100:3 for separation of FAME with up to 3 double bonds, and hexane-acetone-ethanol (100:3:1, v/v/v) for separation of TAG. So far, at least in our hands, the selectivity of the separation is acceptable for unambiguous identification of FAME and TAG classes (see below). As is shown on the pictures (Fig. 3A, 3B), we develop the plates in simple 24 × 5 cm cylindrical chromatography tanks covered by a petri dish. Single development of 4 × 10 cm plates takes about 15 min. Note: The form and the dimensions of the developing vessel are not mandatory; we advise to develop the plates in glass vessels with dimensions close to that of the plate; a height of 2 to 4 cm higher than the upper edge of the plate is recommended to improve the separation. Any laboratory beaker or commercial rectangular chamber with suitable dimensions can be used. Note: Covering the development vessel and shading it from light (except in case from direct sunlight) are not essential.

A

B

CFigure 3. Development of 4 × 20 cm (A) and 4 × 10 cm (B) pre-coated plates; 4 × 20 cm plates ready after development (C).
7. Detection
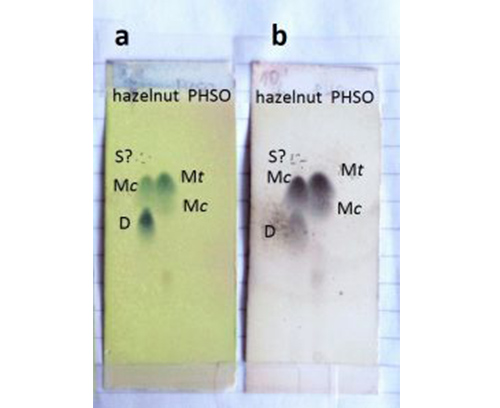
Detection is the critical moment when working with pre-coated plates and especially those with an aluminium back. Detection of lipids, FA and TAG in particular, is troublesome in general because of the lack of chromophores in the molecule. The non-destructive fluorescent reagents, widely used in preparative Ag-TLC, are not suitable in analytical mode because of the low sensitivity (note the low sample load mandatory in analytical Ag-TLC). Sensitive but destructive reagents like sulfuric or phosphomolybdic acids (50% and 10% solutions in ethanol, respectively) are the most common (Note: Both are hazardous!). The plate should be sprayed as thoroughly as possible (fume cupboard!) and heated (any kind of thermostated hot plate will do) at 160-180°C. The result is clearly visible spots/bands of the components with good contrast to the background (Fig. 4A-C).

A
B

CFigure 4. Detection of FAME (A, B) and TAG (C) on commercial aluminium-backed pre-coated silica gel 60 plates after heating on thermostated hot plate at 160-180°C for a few seconds; spraying reagents: 50% ethanolic sulfuric acid (A, B-b, C) or 10% ethanolic phosphomolybdic acid (B-a). Separation of FAME on 4 × 20 (A) and 4 × 10 cm (B) sheets is compared. S, M, D and T denote saturated, monounsaturated, diunsaturated and triunsaturated FAME or fatty acyl moieties, respectively; c and t indicate cis and trans double bonds, respectively.
Heating the plates is required for the visualization of spots to occur. At the temperatures shown, sulfuric acid oxidizes organic compounds to carbon to give black spots on a pale background. Spraying the plates with 10% ethanolic solution of phosphomolybdic acid (yellow-greenish colour) and heating at the same temperatures (Fig. 4B-a) results in the appearance of bluish spots on a yellow background. The colour change is due to a redox reaction between the reagent and the organic substance in the spot resulting in reduction of Mo(VI) to a lower oxidation state. Heating should be carried out carefully, however. Increasing the heating time or heating temperature leads to rapid damage of the layer. The spots are indeed more intensely coloured but the background is damaged to an unacceptable level. The same is true when using phosphomolybdic acid as dyeing reagent – the spots colour to nearly black but the background is intensely stained in bluish black, resulting in seriously troublesome identification.Note: Irrespective of the staining reagent, saturated FAME are either only slightly visible or not at all. Note: Saturated components are detectable at higher temperatures (200°C for example), but this causes rapid (in seconds) darkening of the background and strongly hampers detection. Note: Heat the plate for a few seconds only at 160-180°C.
As expected, components are better resolved on 4 × 20 cm sheets (compare Fig. 4A with Fig. 4B) and these are strongly recommended for identification of TAG. For identification of components in relatively simple FAME mixtures (as in common edible fats and oils), 4 × 10 cm sheets provide good results and are especially suitable for rapid unambiguous detection and identification of trans-FAME. Pre-coated plates treated as above to detect FAME and TAG can be kept almost indefinitely in the laboratory documentation. Detection with both sulfuric acid and phosphomolybdic acid is concentration dependent and could be used for densitometric quantification of components.
In principle, non-destructive detection of fatty acids and triacylglycerols on pre-coated analytical plates is possible with certain limitations. Of these, the most suitable is iodine - the most common nonspecific nondestructive detection reagent for lipophilic compounds (although it can react slowly with polyunsaturated fatty acids). The best way to perform the procedure is simply to place the plate in a closed glass vessel containing a few iodine crystals in the bottom and leave it to be saturated with iodine vapour until dark brown spots on a white background appear within a few minutes (fume cupboard!). Detection with iodine vapour is sufficiently sensitive but once the plate is exposed to air the colour fades rapidly to leave no trace and one should rapidly mark the spots (simple pencil will do). Note: Iodine should be used carefully and after preliminary test of eligibility. The reagent attacks the aluminium backing with prolonged exposure and should be used predominantly with pre-coated glass plates. It may react with the binding material, hampering the detection. Common fluorescent spraying reagents like 2',7'-dichlorofluorescein and Rhodamine G, while being efficient in preparative Ag-TLC, are not sufficiently sensitive for application in analytical Ag-TLC. Isotopically labelled lipids can be detected in situ by radiography.
8. Comments on the use of pre-coated plates
The inability to detect the fully saturated components under reasonable heating conditions limits the employment of pre-coated plates for correct estimation of the sample composition. On the other hand, on the 4 × 10 cm sheets cis- and trans-monounsaturated FAME are clearly differentiated (about 20-30 min from application to detection), giving an excellent tool for the rapid and inexpensive detection of the presence of trans-monoenoic FA in a sample.
The poor resolution of TAG hampers the use of the aluminium-backed pre-coated silica gel 60 plates for identification of all components in complex TAG mixtures. The resolution of TAG is greatly improved by decreasing the sample load, but a lower load increases the problem of detection by the procedure presented above.
The plates are suitable for purity checks and identification of FAME and TAG following preparative Ag-TLC.
