Multiple Interactions in Silver Ion Chromatography of Lipids
The Author: Boryana Nikolova-Damyanova, Institute of Organic Chemistry, Centre of Phytochemistry, Sofia 1113, Bulgaria
Unravelling the interactions behind retention and separation of silver ion chromatography is not merely an academic exercise. It is important in that it may lead to further improvements to the methodology (controlling separation and increasing the efficiency) or in suggesting new applications. What we know now is a result of long observation and practice in silver ion thin-layer chromatography (TLC) and silver ion high-performance liquid chromatography (HPLC) and of continuous efforts to explain the observations by using model systems and calculations. Most of the experiments were carried out using fatty acid derivatives as models, and since the acyl moieties in triacylglycerols are responsible for the complexation with silver ions, the conclusions may be considered as generally valid.
The retention mechanism is more complex than a simple interaction with a silver ion and the pi electrons of a double bond. For example, complexes of a chelate type may be formed, and interactions with the solid substrate of the stationary phase must be taken into account. The interactions responsible for retention, and as a result, for the separations may be divided in four major groups and are valid for silver ion chromatography irrespective of the technique used:
A. Retention/separation due to the different strength of the silver ion-double bond(s) complexes [1].
- Experiments with fatty acids and triacylglycerols show that the k' values increase when the number of double bond increases but at a greater-than-proportional value. Thus, the k' of a linoleate derivative is retained more than twice that of an oleic acid derivative, so triacylglycerols with one linoleic acid residue are held more strongly than triacylglycerols with two oleic acid residues although the number of double bonds is the same. The formation of chelate-type complexes between two double bonds and one silver ion has been proposed to be consistent with spectral data showing that Ag+ interacts with at least two ethylenic double bonds in the same or different unsaturated molecules.
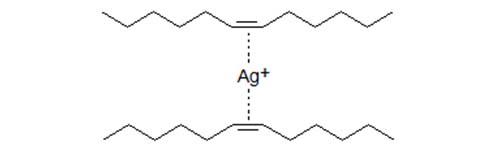
Figure 1. Schematic presentation of Ag+ interaction with two double bonds.
- The weaker retention of allenic and conjugated dienes is due to the weaker complexation between silver ions and the delocalized pi-electrons of these double bond systems
- Fatty acids with double bonds separated by more than one methylene group are retained more strongly than linoleic acid, i.e. with a methylene-interrupted double bond. Amongst C18 dienes, the k' values reach a maximum for the 6,10-18:2 isomer, which is a 1,5-diene system. The same has been observed with diunsaturated hydrocarbons and is ascribed to a distance between two double bonds that favours the formation of a chelate-type complex with a single silver ion.
B. Retention/separation due to the spatial accessibility of the double bonds [1-3].
- Retention is strongly affected by the configuration of the double bond(s) with trans isomers being retained more weakly than cis isomers. The elution order of polyenoic species is determined by the number of trans double bonds in the carbon chain, and the retention increases with the increasing number of cis double bonds. The stronger retention of the cis-isomer(s) is ascribed to the easier accessibility of the double bond(s) while the trans double bond is hindered sterically by the two neighbouring groups.
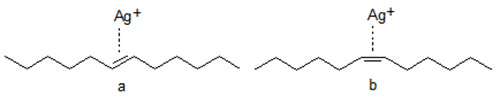
Figure 2. Schematic presentation of the interaction of Ag+ with trans (a) and cis (b) double bonds.
- The separation of regioisomeric triacylglycerols is assumed to depend on the accessibility of the unsaturated acyl moieties, and the assumption is consistent with the experimental results. Experiments with model compounds had shown that positions sn-1,3 are more readily accessible than position sn-2. In the pairs SUS/SSU and SUU/USU the triacylglycerol with unsaturated acyl residue in position sn-2 is retained less strongly (S, saturated; U, unsaturated acyl moiety). In experiments designed to simulate the spatial position of acyl moieties in the glycerol backbone, 1,2-UU cyclohexanediol diesters were held more strongly than were 1,4-UU species (with the same fatty acid composition), supposing that a single Ag+ may form complexes with double bonds positioned in two different closely neighbouring acyl moieties [4]. Experiments to separate regioisomeric triacylglycerols of higher unsaturation (SU2 and, especially U3) are too few at present, and as the identification is ambiguous, conclusions on retention order cannot be made.
C. Retention/separation due to the formation of additional complexes between silver ion and free electrons bearing functionalities [2,3].
The differentiation between monoenoic and polyenoic fatty acids/acyl moieties differing by the position of double bonds in the carbon chain cannot be explained by the interaction between Ag+ and double bonds as a single interaction. Experiments with C18 mono- and dienoic fatty acids show that:
- The retention depends strongly on the position of the double bond in the chain with a maximum at double bond position at the 5-7 carbon atoms. The plot of k' against the double bond position gives a curve of a characteristic form that is close to sinusoidal.
- The k' values are affected strongly by the nature of the ester moiety.
-
The general retention pattern remains the same irrespective of the chromatographic technique used or the type of ester moiety.
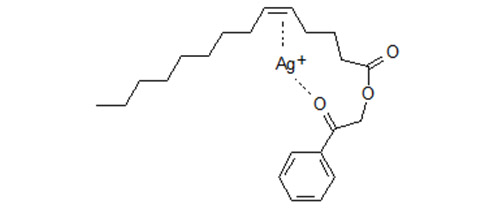
These results are consistent with the assumption that beside the double bond a second reaction site in the molecule interacts simultaneously and that the most likely candidate is the carbonyl oxygen in the phenacyl moiety.
Figure 3. Phenacyl derivative of monoenoic fatty acid. Schematic presentation of simultaneous interaction of Ag+ with a double bond and the carbonyl oxygen of the phenacyl moiety. Phenacyl esters of positionally isomeric monoenoic fatty acids are retained more strongly and are separated much better than the methyl esters [2,3]
The assumption is based on the well-known fact that Ag+ easily interacts with elements bearing free electron pairs such as O, N, and S. Thus:
- A fatty acid is held most strongly when the distance between the two interaction sites in the molecule favours energetically the formation of a three-centre complex, hence the maximum in the retention plot.
- The complexation power of the carbonyl oxygen is affected by the neighbouring substituents in the derivatizing moiety. Electron-donating substituents enhance and electron-withdrawing hamper the complex formation, affecting strongly the retention and selectivity of the resolution.
Similar effects can be expected by modifying the ester moiety with N- and S-bearing substituents, if the strength of these complexes does not outrank by far those with double bonds.
D. Retention/separation due to additional interactions [3].
- Interactions between the analyte and polar/nonpolar sites on the stationary phase are probably responsible for separations according to the chain length/overall polarity of fatty acids and triacylglycerols by silver-ion HPLC. Species were retained according to their increasing polarity with longer-chain hydrophobic components eluting earlier, a phenomenon known as a “normal-phase” effect.
- Interactions between analyte and polar/nonpolar solvents of the mobile phase affect the elution/mobility of the analyte. Mobile phase modifiers such as benzene, toluene and acetonitrile are assumed to interact predominantly with Ag+, competing with the double bonds and displacing the analyte. Isopropanol was found to reverse the retention order of positionally isomeric polyunsaturated fatty acid (C18 and C20 species with 1-3 double bonds) supposedly by affecting the conformation of the molecule. Also, the main component of the mobile phase appears to have a certain effect: hexane-based mobile phases enhance the resolution of fatty acids according to the chain length as expected. While positionally isomeric triacylglycerols are very well resolved with hexane- and toluene-based mobile phases, this has never been achieved with mobile phases based on chlorinated solvents. Of many possible reasons, the different conformation of the molecule might be responsible for this result.
- Polar/nonpolar interaction between stationary phase and mobile phase modifiers like methanol, isopropanol and acetone presumably reduces the interaction between polar functionalities in the lipid molecules and the polar sites in the stationary phase (residual silanol groups mainly) by competing for the latter. Acetic acid is a useful mobile phase modifier in the resolution of free fatty acids presumably by keeping them in a fully protonated form and thus suppressing (to some extent) the interactions with the polar functionalities of the stationary phase.
These interactions act simultaneously and are responsible for useful tuning effects on the selectivity of separation in silver ion chromatography. Systematic investigations are required for a better understanding of their role and the results will provide us with a powerful tool to better control the chromatographic separation.
References
- Nikolova-Damyanova, B. Silver ion chromatography and lipids. In: Advances in Lipid Methodology – One, pp. 181-237 (ed. W.W.Christie, The Oily Press, Ayr, Scotland) (1992).
- Nikolova-Damyanova, B. Silver ion chromatography of lipids. In: Advances in Lipid Methodology – Five, pp. 43-123 (ed. R.O. Adlof, The Oily Press, Bridgwater) (2003).
- Nikolova-Damyanova, B. Retention of lipids in silver ion high-performance liquid chromatography: facts and assumptions. J. Chromatogr. A, 1216, 1815–1824 (2009) (DOI: 10.1016/j.chroma.2008.10.097).
- Nikolova-Damyanova, B., Dobson, G., Momchilova, S. and Christie, W.W. Cyclohexanediol fatty acid diesters as model compounds for mechanistic studies in silver ion high-performance liquid chromatography. J. Liquid. Chromatogr. Rel. Technol., 26, 1905-1912 (2003) (DOI: 10.1081/JLC-120021759).