Mechanism of Retention of Unsaturated Lipids
The Author: Boryana Nikolova-Damyanova, Institute of Organic Chemistry, Centre of Phytochemistry, Sofia 1113, Bulgaria.
In order to use in full the resolution power of silver ion chromatography in lipid analysis, one should have at least a brief knowledge of the basic interactions occurring in the chromatographic system by which lipid molecules are separated according to the number, configuration and position of the double bonds in the fatty acid residues. The two main techniques that should be considered in this context are thin-layer chromatography (Ag-TLC) and high-performance liquid chromatography (Ag-HPLC)
Factors Controlling Retention
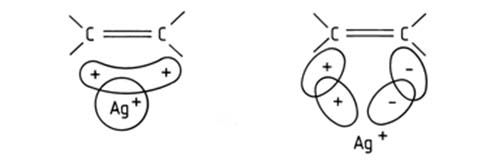
That unsaturated organic compounds, lipids included, form complexes with the ions of many transition metals (Au(I), Pt (II), Ag (I), Cu(I) among others) was known for many years before the principle was applied in chromatography. The nature of the complexation was also reasonably well defined – a charge transfer complex is formed between the metal ion and the pi electrons of the double bond(s) in the unsaturated organic molecule. To be pedantic, the preferred model assumes formation of a sigma type bond between the occupied 2p orbitals of an olefinic double bond and the free 5s and 5p orbitals of the transition metal ion, and a (probably weaker) pi acceptor backbond between the occupied 4d orbitals of the metal ion and the free antibonding 2p pi* orbitals of the olefinic bond [1].
Figure 1. The Dewar model of interaction between Ag+ and an olefinic double bond.
The major advantage of using silver ion (Ag(I)) as complexing agent is that compared to Au and Pt, silver is relatively inexpensive; in contrast to Cu(I), Ag(I) is stable. The salt in widespread use, silver nitrate, is a familiar crystalline compound, stable for a very long period when kept in the dark and easily dissolvable in water, methanol or acetonitrile.
Most important, the complexes are relatively weak, so that the complexation reaction is reversible and is therefore highly suitable for use in chromatographic separation processes.
The successful application of silver ion chromatography for the analysis of lipids (defined as fatty acids and their derivatives) is based on the formation of complexes between Ag(I) and the double bonds located in the fatty acid moiety. Depending on the structure of the fatty acid moieties (chain length, number, configuration and position of double bonds) lipid species are retained for a specific time on the surface of the stationary phase. Long practice has shown that the retention pattern of lipids is governed by the complexation reaction and several simple rules can be outlined. All data obtained so far show that these rules are valid irrespective of the form of the chromatographic technique in use (and also for all unsaturated aliphatic compounds), and these rules were formulated in a review article [2], available online.
-
Retention is determined by the number of double bonds – the higher the number of double bonds, the stronger the molecule is retained in the stationary phase.
-
Of two configurational isomers, one with a cis (Z) double bond is retained more strongly than one with a trans (E) double bond, and the relative retention decreases with the increasing number of trans double bonds in the molecule.
-
Triple bonds form weaker complexes than do double bonds.
-
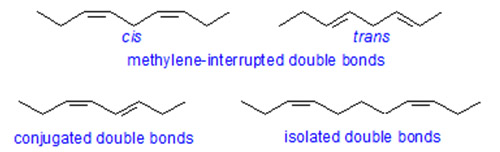
Distance between double bonds affects the retention in that:
Compounds with conjugated double bonds are retained less strongly than those with methylene-interrupted double bonds.
Retention increases with increasing distance between double bonds passing through a maximum with a 1,5-diene system.
-
Retention decreases with increasing chain length of the compound.
Retention Factors
In order to compare the retention of the lipid compounds in a quantitative fashion, some well-defined and widely accepted measurable values are used.
First, a chromatographic separation can be described as an equilibrium process of transferring a given component from the mobile to the stationary phase and vice versa. The respective equilibrium constant K is presented as:
K = Cmob/Cstat
where Cmob and Cstat are the concentrations of the component in the mobile and the stationary phase, respectively. The thermodynamic value K is related to the molar free energy that is associated with the transfer, ΔG, to the gas constant R and to the absolute temperature, T.
In chromatographic practice, it is more sensible and reliable to use a value that can be easily measured and indeed such values were suggested and widely used. In Ag-HPLC, the result of the interactions in the chromatographic system is that a component, X, is retained in the stationary phase for a specific period of time, denoted as retention time, trx. The higher the value of trx the stronger is the retention.
The retention is described by the retention (or capacity) factor k'x, presented as the ratio between trx and the retention time of a nonretained compound to by the equation:
k'x = (trx – to)/to
trx and to can be easily measured with reliable accuracy, and the retention factor allows comparison of the chromatographic behavior of the separated components.
Naturally, there is a close relationship between retention, k', and equilibrium constant, K, presented as:
k' = ψK
- where ψ is the phase ratio (mobile phase/stationary phase).
The retention factor, k', represents essential interactions that occur in the chromatographic system and depends on basic physico-chemical properties of the lipid molecules, the stationary and mobile phases, and nf the temperature.
Separation Factors
What is more interesting from a practical point of view is the ability of the chromatographic system to differentiate between the components of a lipid mixture, denoted as the selectivity of the separation. Retention and selectivity of separation are closely related since solutes are separated on the basis of their different retentions:
α = k2'/k1'
- where α is the selectivity factor, and k2' > k1' are the retention factors of two solutes eluting as adjacent peaks.
In Ag-TLC, the same values are valid. Instead of measuring the retention time, the retention of a specific component is presented by the migration distance on the plate, known as the Rf value. As above, Rf is determined by the ratio between the migration distance of the component, Lx, and the distance from the start to the solvent front (equivalent to an unretained compound), Lo:
Rf = (Lo-Lx)/Lo
The smaller the value of Rf, the more strongly is the component retained.
Selectivity here is presented by the ratio of the Rf values of two adjacent spots.
α = RfA/RfB
In both chromatographic techniques selectivity is presented by a most simple and easily measurable value, the separation factor, R. The practical value of this factor is that it accounts for the width of the chromatographic peaks in HPLC or the area of the spots in Ag-TLC, thus informing for the actual selectivity of the chromatographic separation. The separation factor is presented as:
R = (trA – trB)/(1/2dA+1/2dB)
Where tr is the respective retention time and 1/2d is the half-width of the peaks at the baseline (in HPLC). This information is now derived directly by the software of modern HPLC instruments. And in Ag-TLC -
R = (LA-LB)/(1/2dA + 1/2dB)
Where L is the migration distance from the start to the center of the spot of the component and d is the diameter of the spot. The measurements are performed manually and are probably not very reproducible, but the information is, in general, sufficient to demonstrate the factual separation.
References
- Dewar, M.J.S. A review of πcomplex theory. Bull. Soc. Chim. France, 18, C71-C79 (1951).
- Nikolova-Damyanova, B. Silver ion chromatography and lipids. In: Advances in Lipid Methodology - One, pp. 181-237 (ed. W.W. Christie, Oily Press, Dundee) (1992).