Enzymatic Interesterification
The Author: W. David Cowan, Novozymes UK, 282 Chartridge Lane, Chesham, Bucks. (HP5 2SG), England
Introduction
Enzymatic interesterification is a process in which a lipase enzyme is used to catalyse the exchange of fatty acids attached to the glycerol backbone of a fat. The purpose of the interesterification is to change the physical (melting) properties of the resulting fats to fit them more closely to those desired for food or other purposes.
Fat modification has long been practised in the oils and fats industry because vegetable fats particularly do not have the desired melting properties in their normal state [1]. Hydrogenation, in which unsaturated fats are reacted with hydrogen in the presence of a metal (nickel) catalyst to produce saturated fats, has been available for approximately 100 years. However, to produce fats of the desired melting properties, the hydrogenation is not carried through to completion, resulting in the production of trans-fatty acids. In 1990 it was observed that although trans-fatty acids increase LDL cholesterol to a similar degree to saturated fat, they decrease HDL cholesterol relative to both cis-unsaturated and saturated fats. These initial studies have been followed up by a wide range of investigations where the overall conclusion remains that trans-fatty acid consumption substantially increases the risk of coronary heart disease [2]. For this reason, hydrogenation has largely been abandoned in Western and many other countries and alternative routes for modification of fat properties have been sought.
Interesterification in which fatty acids are exchanged between two fats or within a fat has been used as an alternative to hydrogenation. Initially a chemical catalyst (sodium methoxide) was applied but more recently an enzymatic catalyst has started to replace this older method of fat modification.
The Development of Enzymatic Interesterification
Chemical interesterification using sodium methoxide can be traced back to 1920, when a first patent was granted to Normann [3], but commercialisation only really occurred in the early 1960s when Unilever introduced the process for margarine and other fats [4]. One of the drawbacks of the process was that it randomized the fats and all the fatty acids were moved around without any specific attachment to the glycerol backbone. This may well have prompted the research departments at Unilever, Fuji Oil and Novozymes to investigate lipases as alternative catalysts. Some lipases exhibit stereospecificity working selectively to modify fatty acids in the sn-1,3 position whilst others show no selectivity. The application that received the most attention was the production of a cocoa butter equivalent (CBE) utilising the sn-1,3 specificity of certain fungal lipases. Macrae [5] describes the use of a lipase product absorbed onto a kieselguhr matrix to convert a mixture of palm mid fraction and stearic acid into a CBE-like product containing increased levels of the desired triglycerides. These products could not be produced by chemical interesterification because the particular melting properties of chocolates come from the arrangement of palmitic and stearic acids in the sn-1 and -3 positions and oleic in the sn-2 position. By exchanging some of the palmitic acid on the sn-1,3 position of palm mid fraction with stearic acid a product (CBE) with similar physical properties to cocoa butter could be produced. Because the enzymatic exchange of fatty acids was mainly limited to the outer (sn-1,3) positions of the fat, the oleic acid at the sn-2 position was maintained and the correct fat produced. Chemical interesterification, which randomises the position of the fatty acids, could not produce the desired result.
Immobilising or more correctly absorbing the enzyme onto an inert support allowed the product to function in a nonaqueous environment. At that time all industrial enzyme applications were based on the hydrolysis of water-soluble molecules into smaller ones, and it was generally believed that this was always going to be the situation. In practice the application was not easy to control and required additions of water to maintain the enzyme’s catalytic activity. As the whole system was lipid based, this was not an easy procedure! The enzyme catalyst was also complicated to produce and because the overall demand for CBE was relatively small, there was little incentive to develop and refine the process.
Research continued however and alternative supports for lipases were considered that would be more robust and which might eliminate the requirement for water addition. Ion exchange resins were found to be particularly suitable and several lipases both specific and nonspecific were introduced using these carriers. Novozyme (Lipozyme) 435 has been the most successful of these and a Google search reveals more than 29,000 references to the use of this enzyme. However, the cost of the carrier and the relatively poor yields in production of the lipase enzymes restricted the application to those types of products that can accept the high enzyme cost and for which there was no alternative chemical process.
Two scientific developments altered this position and allowed enzymatic interesterification to develop into a wider range of applications. The first of these was the ability to transfer the gene for enzyme production to a new host microorganism. Modern enzyme production takes place in large vessels under strongly agitated and aerated fermentation conditions. Many desirable organisms for enzyme production cannot tolerate these conditions or have very poor yields. The transfer of the gene to a more robust, high-yielding host strain allows for the economic production of previously uneconomic enzyme products.
This was coupled with the development of a low-cost immobilisation procedure using an inert carrier [6], resulting in a significant reduction in production costs and hence the possibility to extend the range of applications.
Industrial Application of Enzymatic Interesterification
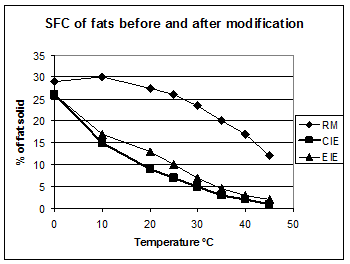
The aim of interesterification is to modify the melting properties of a fat or fat blend to produce a fat suitable for incorporation into margarine or other food fat product. In Figure 1, the solid fat content (SFC) profile of a blend of soybean and fully hardened soybean oil is measured before and after interesterification. Both enzymatic and chemical interesterification produces a softening of the oil. By changing the proportion or the type of fats, different melting profiles can be achieved, according to the application for the final fat product. For example, fats for margarine for use in a warm climate should have a higher melting point than one intended for a cool climate or where air conditioning is used.
Figure 1. Solid fat content (SFC) of a 75% soybean:25% fully hardened soybean oil before (RM) and after chemical (CIE) or enzymatic interesterification (EIE).
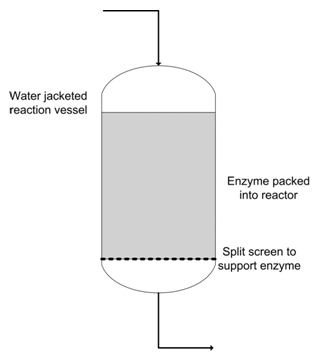
As practised today enzymatic interesterification uses an immobilised lipase held in vertical columns and with the fat blend to be interesterified, pumped down the column. The enzyme catalyses the exchange of fatty acids between the fats in the blend and the resulting product has the desired melting properties. In contrast to chemical interesterification, the enzyme process is a continuous one, with the oil blend being fed into the top of the reactor and continuously removed from the base. The individual reaction vessels have a water jacket to prevent solidification of the fat and the enzyme granules are held on a split screen to prevent them from getting into the oil blend as it leaves the reactor. The shape of the screen as an inverted triangle allows oil to pass through the screen gaps (150 nm), while the larger enzyme particles (average 450 nm) are retained.
Figure 2 shows the layout of a single reactor, containing between 250 and 1000 kg of enzyme. Flow rates through the column are relatively slow, of the order of 1-2 kg oil/kg enzyme/hour. An industrial installation will contain a number of reactors in series to maximise the utilisation of the enzyme catalyst.
Figure 2. Enzyme reactor for interesterification.
If a single reactor is used then as enzyme activity decreases, the flow rate through the column will need to be reduced in order that the required degree of conversion can be obtained. This would result in a decreasing daily output from the interesterification unit, which is not desirable. By operating several reactors in series, enzyme activity will be the lowest in the first reactor in the series and effectively 100% in the last one. The conversion is thus shared along the reactor chain, with ~95% being completed before the oil passes into the last reactor in the line. Eventually there will not be any residual activity in the first reactor and it can be isolated from the others, the old enzyme removed and fresh added. By altering the valves controlling flow, this ”new” reactor can be placed at the end of the line and production continued. If only a single reactor is used then production would have to stop while there was still 10-15% residual activity left in the enzyme column.
The cause of the loss of activity has been studied to determine if it were possible to reduce it and to be certain that enzyme protein was not leaching into the oil. Measurements of protein content in the immobilised enzyme product have revealed that this remains constant even though the measured activity in the enzyme column is decreasing [7]. Also measurements of lipase activity in the oil coming from the column have not shown the presence of active enzyme or enzyme protein, suggesting that the cause of activity reduction is not physical loss of enzyme from the column.
If physical loss of enzyme is not responsible for the observed decrease in activity then other factors must be involved. Oils often contain unsaturated fatty acids which are prone to oxidation and this process can result in oxidation products being present in the oil. Refining is intended to remove these products but it has been established that when primary oxidation products as measured by the peroxide value are too high, then enzyme inactivation will occur. The rate of inactivation is proportional to the PV and at a level of PV > 4, the working life of the enzyme will quickly be reduced to about 50% of the maximum value [8]
However, oxidation products are not the only materials found in oil that can cause enzyme inactivation. While the enzyme is insensitive to the presence of free fatty acids, citric acid or mineral acid residues can also reduce enzyme activity. Oils do contain small amounts of water, generally <0.2%, and this water can dissolve citric acid added as a chelating agent for metals or mineral acids (sulfuric and phosphoric) found in the bleaching earth used upstream of the interesterification process to refine the oil. Uptake of the acids into the enzyme granules results in a lowering of pH and hence a decrease in enzyme activity. The effects of these two components are additive, so for maximum enzyme working life, levels should be minimised.
Analytical methods are available to determine PV and mineral acidity in oil, and the levels can be minimised by the correct preparation of the oil prior to interesterification.
Products Made by Enzymatic Interesterification
The earlier processes for fat modification used high-cost enzymes which limited the application to products also of high value. Based on production in the more recent process, fats modified by enzymatic interesterification have been studied for their quality and functionality in the production of margarines and shortenings. The specific nature of the enzyme-catalysed reaction results in the production of modified fats with higher retention of natural antioxidants, thus reducing the demand for external sources of these for good stability [8].
A second advantage is that the low temperatures involved and catalyst specificity result in products with lower by-product levels and a reduced requirement for purification post-conversion.
Margarine quality has also been examined both for stability and baking performance in several studies and has been shown to be at least equivalent to that of margarines coming from chemical interesterification and in some cases superior.
The process of formulation of fats can follow two approaches. Usually, there will be a specification for a fat based on melting properties. The producer will have a number of raw materials available that can be used for their production. Based on previous studies a selection of the most appropriate can be made and batch laboratory interesterification trials carried out to produce a number of Solid Fat curves. These can then be compared to the desired value of the product in question
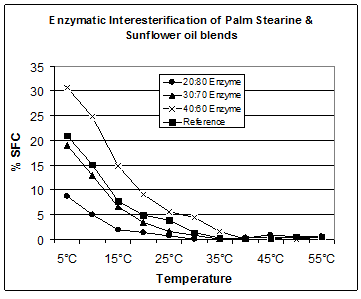
In Figure 3, three blends of palm stearine and sunflower oil have been prepared, interesterified and their solid fat curves compared to the desired product. The 30:70 blend comes closest to the desired product but overall the values are slightly lower than the reference. By adjusting the ratio, to reduce the sunflower component slightly, the curve will move towards the 20:80 blend and match the desired values.
Figure 3. Comparison of enzymatic and chemical interesterification of palm stearine and sunflower oil blends.
Alternatively, if there is already a fat of the correct melting properties made by chemical interesterification, the blend used for that will be the best starting point. As can be seen in Figure 1 the melting properties of fats made by either method are very similar. Typically a laboratory-scale batch interesterification is carried out and the melting properties of the resulting fat determined. Generally there will be small variations from a product made by chemical interesterification due to lower levels of diglycerides in the enzymatically produced fat. By adjusting the ratio between the two components and repeating the laboratory-scale interesterification, the desired melting properties can be obtained. Once the blend composition has been fixed, then this can be used for full-scale production.
Environmental Benefits of Enzymatic Interesterification
The process flow for chemical and enzymatic interesterification is different in that the chemical process is normally a batch operation while the enzymatic one is normally continuous. Comparison of the two is best made by looking at the number of individual process steps involved. Generally we can observe that enzymatic interesterification is a simpler process with fewer steps and lower operating temperatures. The temperature of operation for the enzymatic process is 70°C, while that of chemical varies from 80-120°C according to the implementation of the process.
The chemical catalyst produces a darkening of the oil and some by-products, both of which need to be removed. In contrast the enzymatic process does not result in colour formation and by-products are much more limited.
With a full process description for both systems of production, then a comparison of relative environmental impacts can be made. It is essential that for both procedures, the methods of production of the raw materials, catalysts, production losses and process conditions are described as fully as possible, in order for an accurate assessment to be made. By definition this means that each process must be described by somebody experienced in its operation.
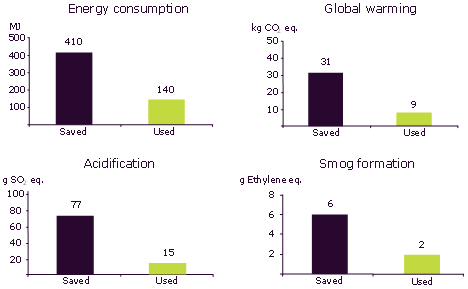
Life cycle assessment of the two interesterification processes for the production of a palm oil-based hard stock for margarine was made according to standard procedures [9]. Four environmental indicators, energy consumption, global warming, acidification and smog formation are used in the analysis process. Modelling of the environmental impact of the enzyme production was made using data generated internally [10]. In Figure 4, the calculated values for amounts saved per ton of margarine hardstock (solid fat) are shown. The method also produces a value for the amounts of each indicator used in the production of the enzyme and related processes. So the true saving is obtained by subtracting the used figure from the saved one. In all cases adoption of enzymatic processing reduces the environmental impact of margarine production and it can thus contribute to a more sustainable production process.
Figure 4. Results of life cycle assessment. Environmental impacts from used and saved materials and energy per ton hardstock produced.
The values obtained are for the particular set of circumstances studied. For example a change in raw materials or a change in operating conditions will result in a different set of values. However, in an earlier study where soybean oil and fully hydrogenated soybean oil were used, there was still a significant saving to be made but in this case the difference between the “saved” and “used” values was greater [11]. This reflected the different energy costs and efficiencies of operating the two alternative processes.
Further Reading
A more detailed explanation of the development of enzymatic interesterification can be found in: wikipedia.
- Gunstone, F.D. Extraction, refining and processing. In: The Chemistry of Oils and Fats, pp. 42-49 (F.D. Gunstone (ed.), Blackwell Publishing, Oxford (UK)) (2004).
- Mozaffarian, D., Katan, M.B., Ascherio, A., Stampfer, M.J. and Willett, W.C. Trans fatty acids and coronary heart disease. New. Engl. J. Med., 354, 1601-1613 (2006).
- Normann, W. (Oelwerke Germania G.m.b.H), Verfahren zur Umesterung von Fettsäureestern, German Patent 417 215 (1925).
- Lampert, D.S. Processes and products of interesterification. In: Introduction to Fats and Oils Technology, pp. 208-234 (R.D. O'Brien, W.E. Farr, and P.J. Wan (eds.), AOCS Press, Urbana, IL) (2000).
- Macrae, A.R. Microbial lipases as catalysts for the interesterification of oils and fats. In: Biotechnology for the Oils and Fats Industry, pp. 189-198 (C. Ratledge, P. Dawson, and J.B.M. Rattray (eds.), AOCS Press, Champaign, IL) (1985).
- Christensen, M.W., Andersen, L., Husum, T.L. and Kirk, O. Industrial lipase immobilization. Eur. J. Lipid Sci. Technol., 105, 318-321 (2003) (DOI: 10.1002/ejlt.200390062).
- Cowan, W.D. and Holm, H.C. Optimization of enzyme efficiency through control of oil quality used in interesterification. Paper presented at the 101st AOCS Annual Meeting, Phoenix, AZ, (2010).
- Holm, H.C. and Cowan, W.D. The evolution of enzymatic interesterification in the oils and fats industry. Eur. J. Lipid Sci. Technol., 110, 679-691 (2008) (DOI: 10.1002/ejlt.200800100).
- Wenzel, H., Hauschild, M. and Alting, L. In: Environmental Assessment of Products. Volume 1: Methodology, Tools and Case Studies in Product Development. Chapman and Hall, London (1997).
- Nielsen, P.M., Oxenbøll, K.M. and Wenzel, H. Cradle-to-gate environmental assessment of enzyme products produced industrially in Denmark by Novozymes A/S. Int. J. LCA, 12, 432-438 (2007).
- Cowan, W.D. Harnessing enzymes for savings. Oils Fats Int., 25-28 (2008).
In This Section
- Marine Oils
- Animal Fats
- Olive Oil
- Palm Oil
- Seed Preparation
- Expanding and Expelling
- Solvent Extraction
- Meal Desolventizing, Toasting, Drying and Cooling
- Introduction to Degumming
- Chemical Degumming
- Enzymatic Degumming
- Alkali Refining
- Optimization of Bleaching Process
- Silica Hydrogel and its Use in Edible Oil Processing
- Deodorization
- Hydrogenation Mechanism
- Chemical Interesterification
- Enzymatic Interesterification
- Solvent Fractionation
- Dry Fractionation
- Hydrogenation in Practice