Retention Order of Fatty Acids and Triacylglycerols
The Author: Boryana Nikolova-Damyanova, Institute of Organic Chemistry, Centre of Phytochemistry, Sofia 1113, Bulgaria
The interaction between Ag(I) and the olefinic double bond(s) in the acyl residue in lipids is the factor determining the retention of the lipid molecule in a chromatographic system. Lipid molecules are retained depending on their structure to give a "retention order", which is of substantial practical interest. With this information, one can predict the order of migration/elution of lipid components under the conditions of silver ion chromatography. These rules apply both to thin-layer chromatography (Ag-TLC) and high-performance liquid chromatography (Ag-HPLC). Stationary phase properties and mobile phase composition may have a modulating effect, but they do not change the general retention pattern.
Fatty Acids
What one observes when a mixture of fatty acids is subjected to silver ion chromatography (after derivatization - most often in the form of methyl esters, although other aromatic derivatives are useful for specific purposes) is that the retention order follows in general the retention rules:
-
Simple fatty acid esters (usually methyl esters) migrate/elute in the order of increasing number of the methylene-interrupted cis double bonds in the carbon chain.
-
Fatty acids with trans-double bonds elute ahead of cis-isomers. The elution order of configurationally isomeric polyenoic fatty acids is determined by the number of trans double bonds in the molecule, and this order holds irrespective of the number and position of the double bonds or the distance between two adjacent bonds or the chromatographic technique in use. For example, for di- and triunsaturated fatty acids the order of retention is cc > ct > tt and ccc > cct > ctt > ttt, respectively. While all-cis and all-trans species firmly hold to this order, it may change for species containing double bonds of mixed configuration, depending upon the mobile phase composition.
-
The distance between double bonds in polyenoic fatty acids impacts the retention order.
(i) Conjugated dienes are retained less strongly than cis-monoenes, irrespective of the configuration and the position of the double bonds.
(ii) Octadecadienoates with isolated double bonds are retained more strongly than octadecadienoates with methylene-interrupted double bonds, with cis-6,cis-10-18:2 (i.e. a 1,5-diene system) retained about four times as strongly as cis-9,cis-12-18:2 (k'' = 10.3), for example. -
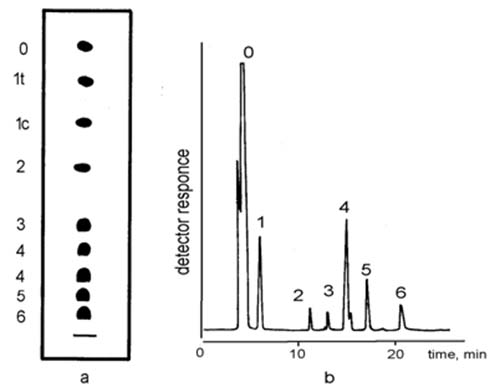
Fatty acids with longer chains migrate/elute ahead of shorter-chain components of the same degree of unsaturation - 18:1 > 20:1; 18:3 > 20:3; 18:4 > 20:4 (see Fig. 1). Separation of fatty acids according to the chain length is not common, however, and is usually not a goal in silver ion chromatography.
Figure 1. Migration/elution order of reference mixtures of fatty acid methyl esters in silver ion chromatography. (a) Ag-TLC, schematic presentation; the figures alongside indicate the number of double bonds ([1], with permission); (b) Ag-HPLC, figures above the peaks indicate the number of double bonds ([2], with permission). 1c and 1t in (a) indicate cis and trans double bonds. Composition of the samples: 16:0; trans-9-18:1; cis-9-18:1; cis-9,cis-12-18:2; cis-9,cis-12,cis-15-18:3; cis-5,cis-8,cis-11,cis-14-20:4; all-cis-4,7,10,13,16-20:5; all-cis-4,7,10,13,16,19-22:6. Note: arachidonic (20:4(n-6)) methyl ester migrates ahead of stearidonic (18:4(n-3)) methyl ester.
-
Additional functionality in the fatty acid chain, such as hydroxy groups or cyclization, may also affect the retention. Based on the ability of Ag(I) to form complexes with oxygen one should expect that unsaturated hydroxyl species will be retained much more strongly than the species with the same chain length and number of double bonds and this was confirmed experimentally [3]. With cyclic fatty acids, retention order depends on whether the double bonds are in the ring or the aliphatic chain [4].
There are some rather unexpected but important experimental issues that are observed when studying systematically the retention of fatty acids. When it became possible to get more accurate and comparable data for the retention in the respective k' values (i.e. when Ag-HPLC became a robust technique), it appeared that retention, "measured" as k' value, does not increase in direct proportion to the number of double bonds, which might be expected assuming that each double bond interacts with a separate single silver ion and that the stabilities of these separate complexes are almost or strictly equal. Instead, the addition of one double bond in 9,12-18:2 leads to about a three times higher k' value than that of 9-18:1; 9,12,15-18:3 is held about 2.5 times more strongly than 9,12-18:2, and 5,8,11,14,17-20:5 is held 1.5 times more strongly than 5,8,11,14-20:4 [3].
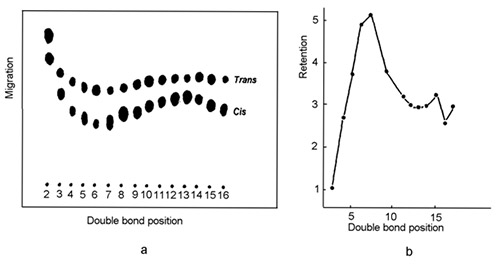
Another observation made in the early days of Ag-TLC and confirmed later with Ag-HPLC showed that the retention order (the migration/elution order, respectively) of a series of 18:1 esters depends on the position of the double bond in the fatty acid carbon chain and that this order holds irrespective of the chromatographic technique in use (Fig. 2).
Figure 2. Migration/elution order of reference mixtures of positionally isomeric octadecenoic fatty acids in silver ion chromatography. (a) Migration order of positionally isomeric cis- and trans-octadecenoic methyl esters in Ag-TLC ([5], with permission). (b) Elution order of positionally isomeric cis-octadecenoic phenacyl esters in Ag-HPLC ([3], with permission). Note that the retention order is the same irrespective of the technique and the fatty acid derivative employed (flip Fig. 2b to find a similar retention pattern as in Fig. 2a).
The most strongly retained species are those with first double bond located at C5-C7 in the fatty acid molecule. The effect was observed for mono-, di- and triunsaturated octadecanoates and eicosanoates with methylene-interrupted double bonds and for octadecadienoates with conjugated double bonds and does not depend on the configuration of double bonds [6].
An important observation was that the effect of double bond position was strongly influenced by the nature of the fatty acid derivatives. Not only the strength of retention but also the differences between the retention factors of the isomers are influenced by the ester moiety thus increasing the selectivity of the chromatographic separation to enable procedures of great practical interest. Series of experiments have shown that the methoxyl group has the weakest effect on retention and selectivity thus minimizing the practical use. Retention and selectivity increases substantially after conversion of fatty acids to phenethyl-, phenacyl- and p-methoxyphenacyl, 2-naphthacyl-, 9-anthrylmethyl- and 2-naphthylmethyl esters. Of these the use of p-methoxyphenacyl esters appears most suitable [6].
Triacylglycerols
The retention order of triacylglycerols follows the general rules outlined above as expected, since the fatty acid moieties are responsible for the interaction with silver ions. Retention behaviour is more complicated, however, since there are three different fatty acid residues to participate in the interaction:
-
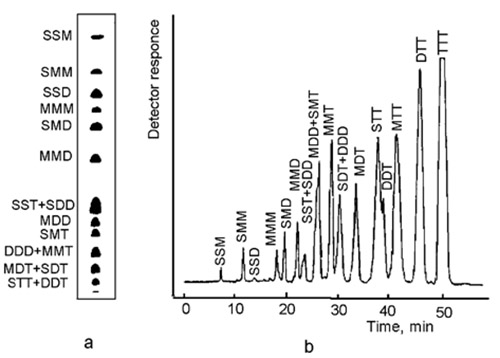
Triacylglycerols are separated according to the overall number of double bonds in the molecule; but of two species with an equal number of double bonds, that in which all or most of the double bonds are concentrated into one fatty acyl moiety is held more firmly like in SMM < SSD, MMD < SDD and other pairs below. The retention order (increasing retention) does not depend on the type of the stationary phase and the chromatographic technique:
SSS < SSM < SMM < SSD < MMM < SMD < MMD < SDD ≤ SST < SMT ≤ MDD < MMT < SDT ≤ DDD < MDT ≤ STT < DDT < MTT < DTT < TTT
where S, M, D and T denote saturated, mono-, di- and trienoic fatty acyl moieties, respectively The notation does not represent the position of the respective acyl moiety in the glycerol backbone (Fig. 3).
The retention order of species with close k' values (denoted as '≤') may change, depending on the mobile phase used.
Figure 3. Migration/elution order of triacylglycerols with up to nine double bonds. (a) Ag-TLC, schematic presentation of a real sample of plant origin, separated on silica gel plate impregnated with 0.5% methanolic AgNO3 ([7], with permission); (b) Ag-HPLC, separation of similar sample on column laboratory-loaded with AgNO3 ([8], with permission). S, M, D and T denote saturated (16:0+18:0), mono-, di- and trienoic (oleic-18:1, linolenic-18:2 and linolenic-18:3) fatty acyl residues.
- The retention order is unambiguous for triacylglycerols with up to nine double bonds and of plant origin. This means that the fatty acid moieties are limited to 16:0, small amounts of 18:0, cis-9-18:1, cis-9,cis-12-18:2, cis-9,cis-12,cis-15-18:3, which limits the variety of triacylglycerols formed. The presence of small amounts or traces of 14:0, 16:1, cis-11-18:1, 20:0, 20:1 or 22:1 fatty acids does not affect the order.
- The retention order of highly unsaturated triacylglycerols (fatty acid residues with 4-6 double bonds and chain length from 12 to 24 carbon atoms, including branched-chain species as in marine lipids, dairy lipids, mushrooms, bacteria) is unclear, although in principle there is no evidence that the general rules do not hold. The problem is that natural highly unsaturated triacylglycerols present very complex mixtures of components with closely related chromatographic properties, indistinguishable by silver ion chromatography at present (and by reversed phase chromatography, by the way). For more details consult [6].
- Triacylglycerols that contain trans double bond(s) elute before the respective cis isomers. For example, SSS < SSMt < SSMc < SMcMt, < SMcMc. Monounsaturated triacylglycerols are retained in the order (increasing retention) EEE EEO EOO OOO (E, trans-9-18:1, elaidic acid residue; O, cis-9-18:1, oleic acid residue).
- Under optimum conditions, it is possible to differentiate between triacylglycerol species differing in the chain length or the position of the double bond in one or more monounsaturated acyl residues. Thus, the species in the MMM class of meadowfoam seed oil were retainedin the order (5-20:1)2,11-20:1 < (5-20:1)3 < 5-20:1,5-20:1,13-22:1, leading to clear separation of these species. Triacylglycerols containing α- and γ-linolenic acids (cis-9,cis-12,cis-15-18:3 and cis-6,cis-9,cis-12-18:3, respectively) are retained more strongly the higher the number of α-linolenic acid residues in the molecule [6].
In relation to the retention of triacylglycerols, there is one further issue that deserves attention since it does not follow directly from the retention rules. Silver ion chromatography allows differentiation between positionally isomeric species. These are species that differ by the unsaturation of the fatty acid in position 2 relative to that of the primary positions (according to the Fischer projection) in the glycerol backbone (positions sn-1 and -3 remain indistinguishable). This ability is of practical importance since it allows (a) tracing adulteration of fats and oils in some cases where the matrices or the "impurity" has a specific distribution of fatty acids in the glycerol backbone; (b) control of randomization of natural fats and oils for different commercial purposes and (c) control of the production of specially designed ‘structured triacylglycerols’. This does not require expensive instrumentation other than a TLC plate with silica gel impregnated with not more than 2% methanolic AgNO3 or an HPLC instrument with a silver ion column. For example, the retention order (increasing retention) of commercially important samples, such as fully randomized palm oil, is -
SMS < SSM, SDS < SSD, SMM < MSM, SDM < MSD
(where S, M and D have the same meaning as above but the notation defines the acyl residue in position sn-2 - the middle position in the notation; positions 1 and 3 are not defined).
All reported results agree in that: (i) positionally isomeric triacylglycerols form adjacent pairs with the species in which the unsaturated fatty acid residue is in position either 1 or 3 are retained more strongly. (ii) The higher the unsaturation of the fatty acid residues the smaller is the difference in the retention of the respective triacylglycerol pair. In the this group of triacylglycerol standards chosen to represent the composition of common plant oils, the retention order observed was as follows:
POP < PPO, PLP < PPL, OOP < OPO, PLL < LPL, LLO < LOL, LLnLn < LnLLn
(P, 16:0, palmitic acid; O, oleic acid (cis-9-18:1); Ln, α-linolenic acid residue (18:3(n-3)). While the pair PLL < LPL was fully resolved, LLnLn was only an early eluting shoulder in the joint peak.
The nature of the silver ion technique does not affect the elution order of positional isomeric triacylglycerols.
References
- Nikolova-Damyanova, B. Lipids by TLC. In: Encyclopedia of Chromatography, pp. 483-486 (ed. J. Cazes, Marcel Dekker, New York) (2001).
- Christie W.W. A stable silver-loaded column for the separation of lipids by high-performance liquid chromatography. J. High Resol. Chromatogr., Chromatogr. Commun., 10, 148-150 (1987).
- Nikolova-Damyanova, B., Herslof, B.G. and Christie, W.W. Silver ion high-performance liquid chromatography of derivatives of isomeric fatty acids. J. Chromatogr. A, 609, 133-140 (1992) (DOI: 10.1016/0021-9673(92)80156-O).
- Christie, W.W., Brechany, E.Y., Sébédio, J.L. and Le Quere. J.L. Silver ion chromatography and gas-chromatography mass spectrometry in the structural analysis of cyclic monoenoic acids formed in frying oils. Chem. Phys. Lipids, 66, 143-153 (1993) (DOI: 10.1016/0009-3084(93)90039-6).
- Gunstone, F.D., Ismail, I.A. and Lie Ken Jie, M. Fatty acids, part 16: thin-layer and gas-liquid chromatographic properties of the cis and trans methyl octadecenoates and of some acetylenic esters. Chem. Phys. Lipids, 1, 376-385 (1967) (DOI: 10.1016/0009-3084(67)90015-1).
- Nikolova-Damyanova, B. Retention of lipids in silver ion high-performance liquid chromatography: facts and assumptions. J. Chromatogr. A, 1216, 1815-1824 (2009) (DOI: 10.1016/j.chroma.2008.10.097).
- Tarandjiiska, R. and Nguyen, H. Triglyceride analysis of orange seed oil by argentation thin layer chromatography. Riv. Ital. Sost. Grasse, 65, 489-492 (1988).
- Christie, W.W. Separation of molecular species of triacylglycerols by high-performance liquid chromatography with a silver ion column. J. Chromatogr. A, 454, 273-284 (1988) (DOI: 10.1016/S0021-9673(00)88620-3).