Principles of Silver Ion Complexation with Double Bonds
The Author: Boryana Nikolova-Damyanova, Institute of Organic Chemistry, Centre of Phytochemistry, Sofia 1113, Bulgaria
- A. Introduction
- B. Principles of Silver Ion Complexation with Double Bonds
- C. Silver Ion Thin-Layer Chromatography
- D. Silver Ion High-Performance Liquid Chromatography
- E. Low-Pressure Silver Ion Column Chromatography
- F. Combined Chromatographic Techniques
- G. References
A. Introduction
It is difficult to imagine how lipid chemistry would have developed if silver ion or “argentation” chromatography had not been utilized in analysis. Since its introduction in 1962 [131,194], this separation approach, based on a single property of the lipid molecules - the nature of their unsaturation, has been of enormous importance in the efforts to elucidate the structures of natural and modified lipids of different kinds and origins. Silver ion chromatography has been successfully applied to all lipid classes in every area of lipid investigation, including lipid chemistry per se, the food industry, plant and animal physiology, medicine and pharmacy. It is considered a necessary step in the sequence of chromatographic methods needed to resolve a complex lipid mixture into simpler molecular species.
Silver ion chromatography is performed predominantly in conjunction with two techniques, i.e. thin-layer (TLC) and column chromatography, including both low (or normal) pressure and high-performance liquid chromatography (HPLC). The number of papers dealing with development or improvements of the methodology as well as with applications of all kinds is now enormous. Two excellent reviews discussed the communications on lipids published up to 1972 by Morris et al. [132,134]. Some recent information can be found in a more general review on TLC [74].
B. Principles of Silver Ion Complexation with Double Bonds
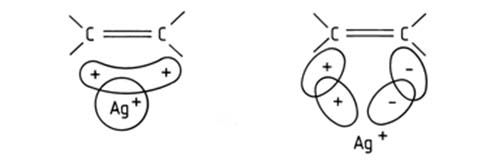
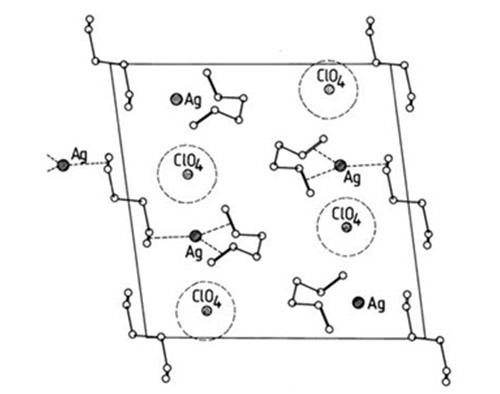
Silver ion chromatography is based on a distinctive property of unsaturated organic compounds, that is, the ability to complex with transition metals, in this instance with silver. The complexes are of the charge-transfer type, i.e. the unsaturated compound acts as an electron donor and the silver ion as an electron acceptor. In fact, the model now considered to correctly represent the bonding [34,64] includes the formation of an s type bond between the occupied 2pπ orbitals of an olefinic double bond and the free 5s and 5p orbitals of the silver ion, and a (probably weaker) π acceptor backbond between the occupied 4d orbitals of the silver ion and the free antibonding 2pπ* orbitals of the olefinic bond [73,76,99,104,120] (Fig. 1). It has been found that silver ions form a bona fide complex with two ethylenic molecules, Ag+(C2H4)2 [104]. X-ray studies of crystalline silver ion complexes with some diolefins showed that Ag+ was coordinated with two double bonds from different olefinic molecules [73] (Fig. 2).
Figure 1. The Dewar model of interaction between a silver ion and an olefinic double bond [132]. (Reproduced by kind permission of the author and of the Journal of Lipid Research, and redrawn from the original).
Figure 2. The crystal structure of the silver perchlorate-1,5-hexadiene complex [73] (Reproduced by kind permission of the Gmelin Handbuch der Anorganischen Chemie and redrawn from the original).
Complex formation between double bonds and silver ion has been and still is investigated intensively, since these complexes play an important role in organometallic chemistry. A great deal of information is now available and has been reviewed [73,76,99,120]. However, quantitative data (e.g. equilibrium constants) exist only for a number of short-chain mono- and diolefins. The general conclusions reached so far can be presented as follows:
- Unsaturated acyclic and carbocyclic compounds form more stable complexes than do aromatics.
- The stability decreases with the increasing chain length.
- The stability decreases with an increasing number of substituents at the double bond in the order R·CH=CH2 > R2C=CH2 > cis R·CH=CH·R > trans R·CH=CH·R > R2·C=CH·R > R2C=CR2. The greater stability of the cis-isomer can be ascribed either to the relief of strain when the complex is formed or to steric hindrance by the two bulky groups when they are in a position trans to each other [120]. Conjugated polyenes form less stable complexes than do those with methylene-interrupted double bonds, and the stability increases with increasing distance between the double bonds, perhaps because a chelate complex can be formed in the latter case.
- The stability increases when a hydrogen atom from a molecule of the R·CH=CH·R type, for example, is replaced with deuterium or tritium; the effect has been ascribed to greater electron release from a C-D than from a C-H bond [120].
Generally, the rate of complexation is very rapid, but the complexes are unstable and exist in equilibrium with the free form of the olefin. The coordination forces seem to be very weak [>76] and the infrared spectrum, for example, shows very little shift in frequencies from those of free double bonds [73]. These particular properties of complexation between a double bond and a silver ion are favourable for use in chromatography, and they enable the performance of the different silver ion chromatographic techniques developed so far. Indeed, most of the studies on the stability and properties of complexes that provided the above rules were performed using chromatographic methods, gas chromatography (GC) mainly [120]. The same properties enable silver ion chromatography of lipids also.
When passing from simple short-chain mono- and diolefins to unsaturated lipids, the problems become much more complicated. In the simplest case, the unsaturated molecule may be represented by a long-chain (16-22 carbon atoms) fatty acid with one to six double bonds, which can be of the same or mixed configurations (i.e. either cis (Z) or trans (E) or both); the carboxyl group may be derivatized to a methyl or other ester moiety. Triacylglycerols and glycerophospholipids contain a variety of fatty acyl residues with different chain lengths and numbers of double bonds (for practical reasons, the highly polar phosphorylated end of a glycerophospholipid is usually replaced by a less polar acetyl or aromatic group for analysis (see Section C.4 and Section D.4). Obviously, there are then many factors that can affect the complexation with silver ions. However, in spite of the complicated character of lipid molecules, the rules presented above have been found to be generally valid.
First, the cis/trans rule operates universally, with cis fatty acid isomers forming stronger complexes than the corresponding trans [132]. The chain-length rule was found to be valid also, with the order of increasing stability being - saturated < trans-22:1 < trans-20:1 < trans-18:1 < cis-22:1 < cis-20:1 < cis-18:1 fatty acids [56]. The double bond position was not specified in this paper, but recently this migration order was confirmed for the cis- and trans-18:1 and 22:1 fatty acids of the (n-9) series [28,129] (See also Section C.2). In fact, it would probably be more accurate to describe the phenomenon in terms of differences in the chain lengths of the alkyl groups attached to the each end of the double bond, rather than overall chain length per se. Monoenes were found to form stronger complexes than monoynes (one acetylenic bond) [131]. Conjugated dienoic fatty acids form weaker complexes than the methylene-interrupted dienes, and for the limited range of isomers studied the stability increased with the increasing distance between the double bonds [134]. Unfortunately, such data are available only for dienoic fatty acids. Replacement of a hydrogen atom with deuterium increases the stability of the complex [180]. With the naturally occurring fatty acids with methylene-interrupted double bonds, the stability of the complexes increases with an increasing number of double bonds in the molecule, although nothing appears to be known of the mechanism of the interaction between silver ions and multiple double bonds [132,134]. Fatty acids with double bonds of mixed configuration complex more weakly the greater the number of trans double bonds. On the other hand, with fatty acids in which both the chain length and the position of the double bonds differ, it is not always easy to predict the order of elution from first principles. Thus, fatty acid derivatives complex in a similar manner in general to the simple olefins and the effects have been found to be valid for all silver ion chromatographic techniques developed so far. More details are presented below in the relevant sections.
The situation becomes more complicated when glycerolipids complex with silver ions. To the author’s knowledge, there are no theoretical or experimental studies on the mechanism of complex formation between silver ions and complicated unsaturated molecules like glycerolipids. Almost nothing appears to be known about the electronic and steric effects in these molecules and their possible influence on the complexation. There is some limited evidence that the complexation can be presented as a simple sum of the complexation abilities of the individual fatty acid residues, which may mean that these lipids do not act as compact molecules [75]. A triacylglycerol molecule, for example, complexes more strongly the greater the total number of double bonds [78]. Yet, of two molecules with an equal number of double bonds, that with the double bonds grouped in one fatty acid residue forms stronger complexes (i.e. 18:0-18:0-18:2 > 18:0-18:1-18:1). Perhaps surprisingly, there appears to be no quantitative information on the nature of this phenomenon for simple molecules. Trans double bonds reduce the stability [194]. Generally, unambiguous data for the order of triacylglycerol complexation exist only for molecules with up to nine double bonds (i.e. trilinolenin), and there is only one paper in which the behaviour of more highly unsaturated molecules is partially described [116]. The chain length of the fatty acid residues may have some effect, but again no data are available. On the other hand, the position of the unsaturated fatty acid in the triacylglycerol molecule was found to affect the complexation in some chromatographic systems [20]. Thus, an SMS triacylglycerol, i.e. with a monoenoic acid in position 2 and saturated components in the primary positions, was found to complex less strongly than SSM, perhaps because of steric hindrance of the unsaturated fatty acid residue when placed in this position [42]. However, the nature of the effect varies for different molecular species (see Section C.3).
With diacylglycerol derivatives, the effect is less complicated since they possess only two fatty acyl residues. The same factors affect the complexation, but the influence of the chain length and double bond configuration of the fatty acid is more clearly defined, while steric factors are probably less important [147].
When considering silver ion complexation in the context of a chromatographic system, another factor must be also considered, that is, the role of the supporting material. The most widely used support material, silica gel, possesses appreciable polarity and absorption activity. Therefore, the migration or elution order of lipids cannot be ascribed to the complexation reaction with silver ions and double bonds only, although it is clear that this is usually the major factor. The retention of a lipid molecule in any such system must be a result of a mixed retention mechanism [77,156]. For example, an unsaturated fatty acid methyl ester has been assumed to complex with the silver ions through its double bond(s) and to interact with the silanol moieties through its methyl ester group [77]. Depending on the position of the double bond, the molecules will have a different conformation, and those molecules may be held more strongly in which the distance between the double bond and the ester group fits better the distance between the silver ion and the silanol moiety. These two interactions have been suggested in explanation of the specific migration patterns of different positional isomers of fatty acid methyl esters, when complete series of octadecenoates [77], octadecynoates [21], octadecadienoates [41] and octadecadiynoates [119] have been co-chromatographed on a single silver ion TLC plate (for more details see Section C.3).
A separate but related problem is the topology of silver ions on the adsorbent surface. (Approaches to the incorporation of silver ions in chromatographic systems are discussed for each technique below). For example, in an interesting investigation of silver ion TLC [111], it was found that part of the silver nitrate remained in crystalline form filling the pores of the silica gel after drying the plate to activate it. On the other hand, a further proportion of the silver nitrate remained dissolved in the water that is always bound to silica gel. The aqueous silver nitrate was assumed to be responsible for complex formation. Saturation of the silica layer with water prior to separation has even been proposed in order to obtain a “pure” complexation reaction [187], but such an approach does not appear to have been tried with lipids. If, however, the excess of silver nitrate does indeed remain in a crystalline form and does not take part in complexation, impregnation of an adsorbent in a TLC plate or column with a highly concentrated solution of silver nitrate is of no practical value. Some of the results obtained with silver ion TLC seem to confirm this observation (see Section C.1 and Section D.1).
Similarly, mixed retention mechanisms cannot be excluded when macroreticular resins or silica-based ion-exchangers in the silver ion form are used with HPLC or low-pressure columns. Silica-based ion-exchangers may even present the more complicated case, since there may be some free silanol groups together with bonded organic moieties such as propylphenyl attached to the sulfonic acid salt of the silver ion; there is no information available on the relative contributions of any of these groups to separations. However, from those results reported so far, it seems that the effect of the complexation with the double bond is again predominant. It is not clear whether a mixed retention mechanism improves or hampers the silver ion complexation, since there have been no systematic quantitative studies of such phenomena.
When discussing mixed retention mechanisms, it is necessary to consider the mobile phase, which is also an important component of a chromatographic system. The proper choice of the solvents determines the selectivity of the separation and the migration of the different species to an appreciable extent. In practice, the mobile phase may interact both with the solute and the support material. There are no systematic data available so far, but it has often been noted that better resolution in silver ion chromatography is achieved by using chlorinated solvents as major components of the mobile phase. Numerous examples are discussed in the following sections.
Finally, a few attempts only have been made to present the complexation or chromatographic retention of lipids in silver ion chromatography in quantitative terms. In each instance, silver ion TLC was the method used. First, Gunstone and Padley [78] proposed arbitrary values for what they called the “complexing power” of 18:0, 18:1, 18:2 and 18:3 fatty acids, namely 0, 1, 2+2a and 4+4a, where a < 1, i.e. the increase in the “complexing power” values was greater than the increase in the number of double bonds. The effect was first observed by Scholfield et al. [173]. Later, Grinberg and Ceglowska [75] introduced the concept of the “relative force of complex formation” of a fatty acid molecule, presenting it as:
f·ci = [(1-Rf)i - (1-Rf)s] / [(1-Rf)o - (1-Rf)s]
where Rfi is the Rf value of a given unsaturated fatty acid, and s and o denote the same values for stearic and oleic acid, respectively. The f·ci values increase with increasing unsaturation, with that of oleic acid being defined as 1.0, since this acid was used as an internal standard. Recently, Pchelkin and Vereshchagin [151] proposed a modified empirical expression, calculating the “relative polarity” of the fatty acids through the Rf values of selected monoacid sn-1,2-diacylglycerols, dipalmitin (SS), diolein (OO) and dilinolein (LL); 1,3-dilinolein was used as an internal standard. The ”relative polarity” was expressed as
pi = ln [(hR1,3LL)SS]/[(hR1,3LL)UU]
where hR = 100 x Rf of a given diacylglycerol relative to the 100 x Rf value of the internal standard and UU (diunsaturated) denotes either OO or LL. The values for the “relative polarity” found in this work were very close to those established earlier, namely, 0, 1.0, 1.9 and 5.1 [75] and 0, 1.03, 2.46 and 5.45 [151] for 18:0, 18:1, 18:2 and 18:3, respectively. In all three papers [75,78,151], it was assumed that the respective values for the tri- or diacylglycerols could be expressed as a simple sum of the values for the fatty acyl residues, i.e. the contribution of a fatty acid in a complex lipid molecule is not affected by the strength and properties of the silver ion complexes with neighbouring fatty acids in the same molecule. Such models are too simple and do not take account of the steric factors which, as discussed above, may affect the complexation of a triacylglycerol molecule especially. However, the model proposed by Pchelkin and Vereshchagin [151] may sufficiently describe the behaviour of diacylglycerols in silver ion TLC.
The task of each chromatographic procedure is to fully expose all structural differences between molecules in order to obtain as complete a separation as is possible. Since a complexation reaction governs the separation, it could be assumed that better results might be obtained under a deficiency of silver ions rather than with an excess. The mobile phase should be of moderate or even low elution power with carefully selected modifiers which can compete either for the polar sites on the support (e.g. methanol) or for the silver ions (e.g. acetonitrile).
The actual performance of the main silver ion chromatographic techniques and the results achieved are described in the sections that follow.
Abbreviations
FAME, fatty acid methyl esters; FID, flame-ionization detector; GC, gas chromatography; HPLC, high-performance liquid chromatography; MS, mass spectrometry; RP, reversed-phase; SPE, solid-phase extraction; TLC, thin-layer chromatography. With molecular species: S, M, D, T, Te, P and H denote saturated, monoenoic, dienoic, trienoic, tetraenoic, pentaenoic and hexaenoic acyl moieties, respectively.
Nikolova-Damyanova, B. Silver ion chromatography and lipids. In: Advances in Lipid Methodology - One. pp. 181-237 (1992) (Ed. W.W. Christie, Oily Press, Ayr). Published here by kind permission of P.J. Barnes & Associates (The Oily Press), who retain the copyright.