The Use of Countercurrent Chromatography (CCC) in Lipid Analysis
The Author: Walter Vetter, GC/MS chromatogram of the trimethylsilyl ether of campestanol
Counter-current chromatography (CCC), frequently referred to as high-speed counter-current chromatography (HSCCC, see also below), is a separation technique based on the distribution of analytes between two immiscible liquid phases, which is performed in a long and narrow tube. The main advantage of CCC is its high sample capacity paired with a low consumption of solvents. To give an example, analytical to semi-preparative CCC systems enable the separation of ~500 mg sample with ~100-500 mL solvent. Linking the separation power of chromatographic methods with the (semi-)preparative performance of CCC generates a strong tool suited for the isolation of natural products, for the purification of raw products and for the fractionation of complex samples, i.e. topics which are all relevant in lipid analysis. Many outstanding papers have been published in the field of CCC, but there is one paper everyone – from beginner to expert – should have read. This paper by the inventor of modern CCC, Professor Yoichiro Ito, summarizes the golden rules and pitfalls in CCC [1].
Principle of CCC
Invented around 1970 [2], the principle of CCC is related to the liquid-liquid-extractions in a separation funnel, in which a compound is distributed between the two immiscible liquid phases (Figure 1a). In CCC, one phase is kept stationary while the other, mobile phase is pumped through a long and hollow tube and serves to transport the analyte(s) through the system. During this transport, CCC takes advantage of thousands of such liquid-liquid-partitioning procedures, so providing the separation power expected from a real chromatographic technique.
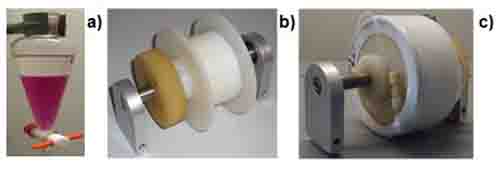
Figure 1: Photographs of (a) liquid-liquid extraction in a separation funnel with a biphasic solvent system in which the colorant is exclusively diluted in the lower phase, (b) an unwound CCC coil and (c) a CCC coil wound up with PTFE tubing. In CCC, thousands of “liquid-liquid extractions” on the way through the coil lead to the separation of compounds similarly soluble in both layers of the biphasic system.
The long and hollow tube is typically made of PTFE, which is wound multifold around a coil (or bobbin) (Figure 1b,c). Typically, two or three of such helical “multilayer coils” are serially connected and integrated into a centrifuge in such a way that they rotate in a planetary motion in a particular arrangement, which avoids any twisting of the tubing connections [1]. During the separation, the coils are rotated at speeds of ~1000 rpm. During the CCC run, permanently changing gravitational forces are acting on the mobile and the stationary phase, resulting in frequent mixing and separation (= demixing) processes in the separation tube.
The introduction of the high-speed centrifuge system has led to the term “HSCCC”. Nowadays this mode is more or less explicitly used so that there is no more an apparent need to distinguish high-speed CCC from CCC. As a consequence, “HSCCC” is more and more substituted with “CCC” in the scientific literature (similarly to the use of “GC” instead of “HRGC” for capillary or high resolution gas chromatography).
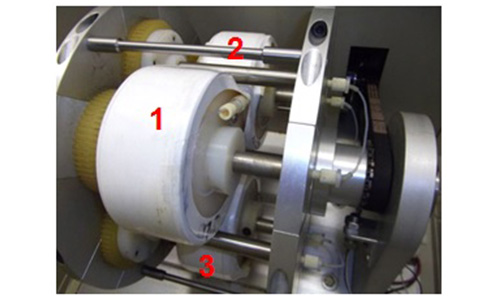
Figure 2: Photograph of the inside of a commercial CCC centrifuge with three serially connected multilayer coils labelled 1,2,3. During the CCC run the coils are rotated at ~1000 rpm.
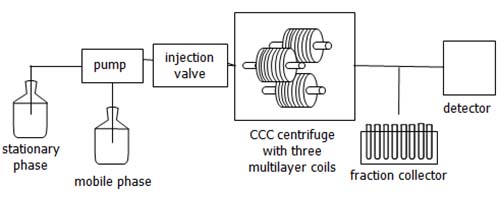
Aside from the CCC centrifuge, modern benchtop CCC systems are constructed in the same way as HPLC systems. It is just replacing the HPLC column with the CCC centrifuge unit (Figure 3). Typically, a CCC system consists of a solvent reservoir, (HPLC) pump, injection system, detector and fraction collector. Currently, there are only a few constructors of commercial CCC systems, but these systems are suitable for the different problems that can be solved by CCC.
Figure 3: Schematic setup of a CCC system (modified from reference [14]). The equipment is the same as in an HPLC setup except that the HPLC column is substituted with the CCC centrifuge (Figure 2).
Factors having an impact on the CCC separation
The solvent system consists of two immiscible phases, one of which is being used as the stationary phase and the other one as the mobile phase. The search for a proper solvent system for the target compound(s) is the most important preparatory work for a successful application of CCC. A suitable solvent system should meet the following requirements [1]:
-
The selected solvent components need to form two immiscible phases.
-
The analytes should be stable and soluble in the system.
-
The solvent mixture should form an acceptable volume ratio of the two phases, to minimize the solvent loss.
In a properly selected solvent system, the analytes should show a reasonable distribution coefficient KU/L (Equation 1):
| Equation 1 |
KU/L is the concentration ratio CU/CL of a compound in the same volume of the two phases (i.e. the distribution coefficient). For a successful application of CCC, the KU/L value of the target compound should preferably be between 0.5 and 2.0. KU/L values should be determined in advance by shake-flask experiments using the following (or a similar) procedure:
-
Place an aliquot of the analyte (dry) in a 6 mL derivation tube and add 2 mL from each phase of an equilibrated solvent system (solvent systems should be made ~24 h before the use).
-
Close and shake the tube and wait for phase separation.
-
Take an aliquot of both phases for determination of the concentration of the analyte(s), e.g. by GC or HPLC [1].
With the elution of the mobile phase, the analytes are the faster eluted the less they are miscible in the stationary phase. The mobile and stationary phase can substitute one another, which means that with the same solvent system CCC separations can be performed either in normal phase and reversed phase mode. In normal phase mode (“tail-to-head”), the upper (nonpolar) phase serves as the mobile phase while the lower (polar) phase is being used as the stationary phase. KU/L values from shake-flask experiments indicate the preferred mode of action for a successful separation with CCC. Compounds showing KU/L values <1 should be preferably analyzed in reversed-phase mode (“head-to-tail”). Vice versa, the normal-phase mode (“tail-to-head”) is recommended for compounds with KU/L > 1 (Table 1). To give an example: in normal phase mode, long-chain saturated fatty acids are eluting first. In reversed phase mode (“head-to-tail”), the upper (nonpolar) phase serves as the stationary phase while the lower (polar) phase transports the analytes through the CCC system. In this mode, long-chain saturated fatty acids are eluting last (Table 1).
| Table 1: Normal and reverse phase modes in CCC with selected characteristics. | |||||
| Mode | CCC expression | Best suited for KU/L |
Stationary phase | Mobile phase | Long chain saturated fatty acids… |
| normal phase | “tail-to-head” | 1 – 2* | Lower (polar) | Upper (nonpolar) | …elute before shorter ones |
| reversed phase | “head-to-tail” | 0.5 – 1 | Upper (nonpolar) | Lower (polar) | …elute after shorter ones |
| * switching to “tail-to-head” mode corresponds with (reciprocal) KL/U values of 1-0.5, i.e. the desired range of K values for CCC separations | |||||
Development and selection of an appropriate solvent system for CCC separations
The polarity of the analyte is a key-factor to be considered for the successful isolation of analytes when selecting a biphasic solvent system in CCC. Nonpolar analytes require a biphasic nonpolar solvent system because good solubility of the analyte in the solvent system is essential for large-scale fractionation in a single run. The rotation of the coil system usually increases the temperature by around 5° C [3]. Therefore, solvent systems should be tested for temperature stability when the CCC system does not feature a tempering tool [3]. Performance at different temperatures will also affect the phase solubility ratios, and in the worst case, the solvent system will not separate into two phases, so this parameter should be taken into account.
The settling time (i.e. the time required for phase separation after shaking) of the solvent system should be less than 20 s because longer setting times decrease the retention of the analytes in the stationary phase [1]. The larger the difference in the KU/L values of two compounds, the better they can be separated from each other [1]. For lipid compounds, alkanes such as n-hexane, n-heptane and/or cyclohexane are the primary choice for the upper phase, whereas the lower phase may contain acetonitrile, methanol, ethanol, low amounts of water, and many others. Frequently, some 20 solvent mixtures have to be tested in such shake flask experiments.
Evaluation of the solvent system and pre-determination of the elution volume
In order to be independent of lab-to-lab and run-to-run variations, a standardization procedure has been suggested [4]. Individual CCC fractions were related to the total volume of mobile phase (Vm), which has passed the coil at the end of each fraction. From this volume, the extruded volume of stationary phase is subtracted to give the corrected volume. Division of the corrected volume by the total coil volume allows predicting the elution of a substance from the CCC (Equation 2):
| Equation 2 | |
| - with EV = corrected elution volume in percent; Vm = Volume of mobile phase; Ve = volume of extruded stationary phase; Vc = total coil volume [4]. | |
With the help of equation 2, each CCC fraction can be expressed as a corresponding EV value. Accordingly, the elution of a compound (e.g. a fatty acid) from the CCC system can be described in relation to these values, especially for KU/L values near the recommended range of 0.5-1 [1]. For instance, the methyl ester of 14:0 showed an EV value range from 32.0 to 48.9% (mean EV 40.5%), which agreed well with the KL/U = 0.408 [4]. This measure facilitates the comparison of CCC fractionations with both different fraction volumes and different coil volumes (when using the same solvent system).
It should also be noted that solvents are not completely immiscible with each other. Even stable two-phase systems do not completely separate polar and nonpolar solvents. Low amounts of n-hexane are also soluble in aqueous solutions and the same applies the other way round (Table 2).
| Table 2. Percentage composition of n-hexane, cyclohexane, tert-butyl methyl ether (TBME), methanol and water in the upper and lower phase of the solvent systems n-hexane/TBME/methanol/water (5/2/5/3) [5]. | ||
| Upper phase | Lower phase | |
|---|---|---|
| n-hexane | 80.5 | 0.4 |
| TBME | 18.9 | 11.8 |
| Methanol | 0.4 | 57.3 |
| Water | 0.2 | 30.5 |
This and other issues lead to the fact that the stationary phase is not entirely kept in the system, but aliquots are transported with the mobile phase out of the system (i.e. a kind of column bleed or flooding of the stationary phase). In order to achieve a good chromatographic resolution a minimum of 65–70% of the stationary phase should remain in the column. The more of the stationary phase that remains in the system, the better the separations that can be achieved.
Challenges in the use of CCC in the field of lipids
Lipid compounds (fatty acids and components of the unsaponifiable matter) are characterized by being soluble in organic solvents and immiscible in water. For this purpose, most lipid compounds can be effectively enriched in a separation funnel in a two phase system consisting of n-hexane/water. Lipids will rather exclusively move to the organic n-hexane phase. That means, these compounds have partitioning coefficients between the n-hexane and aqueous phase of KU/L >100 (see Figure 1a). Such situations do not require the use of CCC which is powerful at KU/L = 0.5 – 2 (Table 1). This is where the problem arises, i.e. the finding of two immiscible liquid phases that distribute lipid compounds in about the same ratio, and with high concentration.
Application of CCC for the separation and isolation of fatty acids
Fractionation and isolation of specific fatty acids from natural sources (e.g. oils) is an important task which can be managed by CCC. Liquid chromatographic separations of fatty acids are governed by the so-called equivalent chain length (ECL) rule, which has initially been developed for GC separations (Equation 3):
| Equation 3 | |
| - where N is the number of carbon atoms n is the number of double bonds in the fatty acid [6]. | |
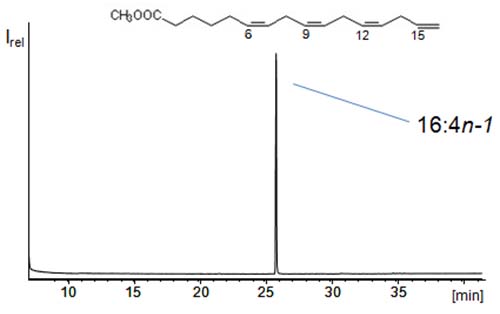
The ECL rule compares the elution of different fatty acids and says that one double bond is equal to two carbons. One prominent example for fatty acids having the same ECL is 16:0 and 18:1n-9. Both major fatty acids have an ECL of 16, that means they are difficult to separate by liquid chromatographic methods. If this thought is pursued, polyunsaturated fatty acids (PUFAs) share the ECL with short-chain saturated fatty acids. For instance 22:6n-3 has an ECL of 10 (i.e. the same ECL as 10:0 and 12:1 isomers). However, 10:0 and 12:1 are rarely found (together with 22:6n-3) in samples. For this reason, CCC offers excellent opportunities for the isolation of PUFAs from marine oils. Accordingly, it was a rather simple task to isolate the methyl ester of 16:4n-1 (ECL = 8), i.e. a PUFA with the fourth double bond on the terminal carbon, from the fatty acid methyl esters obtained from fish oil (Figure 4) [7]. A simple two-phase mixture of n-hexane/methanol/water (~160/80/1) could be used for this purpose in tail-to-head mode (Table 1). However, 22:6n-3 and 20:5n-3 (ECL = 10) could not be separated under these conditions. Stepwise modification of a simple two phase system enabled Bousquet and Le Goffic to separate the (free) PUFAs 22:6n-3, 20:5n-3, 18:4n-3, and 16:3n-3 (all ECL = 10) from each other [8]. This was achieved by the use and stepwise increase of low amounts of water in n-heptane/methanol. Without water, the four PUFAs showed the same KU/L values of ~0.45 in n-heptane/methanol. By the addition of 10% water, the KU/L range increased from 0.55 to ~1.2 (22:6n-3). Under these conditions these PUFAs with the same ECL could all be separated from each other [8].
Figure 4: GC/MS total ion scan of the rare polyunsaturated fatty acid 6,9,12,15-hexadecatetraenoic acid (16:4n-1) methyl ester (with a terminal double bond) isolated by means of CCC from a fish oil [7].
Yet, in the field of lipids, the use of water in a two-phase solvent system reduces the solubility of fatty acids so that the injected sample amount has to be reduced. Typically, such drawbacks can be overcome by performing CCC in a two-step mode. In a first step, the whole PUFA fraction is enriched by pre-separation of the undesired compounds (e.g. saturated fatty acids when the goal is the isolation of PUFAs). This can be achieved by using only minute amounts of water [8]. In a second step, the optimum conditions (here: n-heptane/methanol/water, 500/415/85) are used, and higher amounts of the PUFA fraction can be injected [8].
This strategy has also been used in form of a two-stage CCC method based on (1.) an enrichment step followed by (2.) an isolating step [9]:
1. The enrichment step
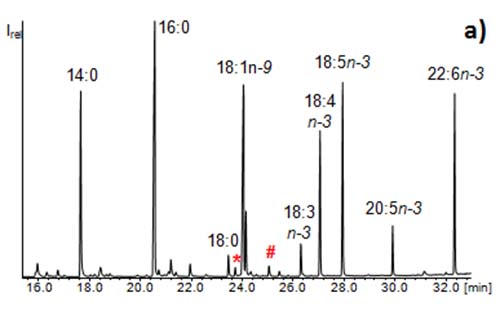
The enrichment step is based on the CCC fractionation of the sample (extraction of for example 30 fractions). These are examined thoroughly by means of GC/MS. Usually GC/MS chromatograms of fatty acid methyl esters (FAMEs) are characterized by the major compound. Minor compounds are only noticeable when present at an abundance of ~1% relative to the dominating compound (Figure 5a). By the CCC fractionation, minor fatty acids in the original sample can become major compounds in the corresponding fraction [4]. As a consequence many more fatty acids than initially detected prior to the CCC run can be identified in the sample (Figure 5b). Due to the good distribution of the compounds in the single fractions, the GC/MS analyses allow the identification of many compounds, which are not detectable without CCC fractionation. This strategy can be used to determine and identify trace compounds in samples. For instance, 430 different fatty acids could be detected in one butter sample (mainly in the filtrate obtained from urea complexation) [10].
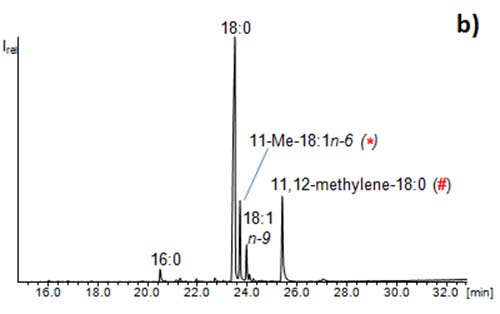
Figure 5: GC/MS chromatograms of (a) fatty acid methyl esters from a microalga and (b) a CCC fraction of the same sample illustrating the enrichment of the methyl esters of the minor fatty acids 11-Me-18:1n-6 (*) and 11,12-methylene-18:0 (#) (“enrichment step”). The full isolation of these rare fatty acids would require a second CCC (“isolation step”).
Such attempts are best performed with FAMEs because the CCC fractions can be directly (or after dilution) measured by GC/MS. In such an enrichment mode, it makes little sense to optimize the separation in the case of complex mixtures of fatty acids because resolution of one pair will be accompanied with the co-elution of another pair.
Figure 5b also illustrates another point. The separation power of CCC lags behind HPLC. Fatty acids usually elute as comparably broad peaks which (in dependence of both the fractionation volume and the amount of the compound) distributed over 2 to 5 fractions. As a consequence, traces of 16:0 already eluted into the fraction of 18:0, and so did 18:1n-9 and other related fatty acids. These separation problems have then to be solved in the second CCC run, i.e. the isolation step.
2. The isolation step
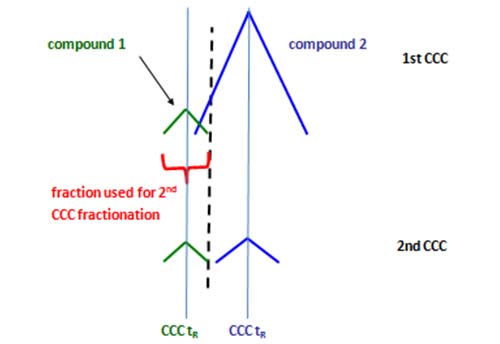
As for other chromatographic systems, the retention (elution) time of a compound from the CCC system is largely independent of the injected amount. However, peaks of highly concentrated compounds are broader and this reduces resolution and separation power. For the isolation of minor compounds, a second CCC run (even with the same solvent system) can help to solve separation problems as illustrated in Figure 6.
Figure 6: Simplified scheme of the repeated CCC fractionation using the same solvent system. In the 1st CCC (top, “enrichment step”) major fatty acids and minor fatty acids can be enriched in individual fractions. When fractions from the 1st CCC containing compound 1 are analyzed in the 2nd CCC (bottom, “isolation step), they can be fully separated from compound 2.
In the upper plot, a minor compound 1 is overlapping with a major compound 2 and compound 1 cannot be isolated in neat form in one run. However, fractions containing compound 1 will contain lesser amounts of compound 2 than in the first run. When these fractions are re-injected, compound 1 can be separated from compound 2 (Figure 6, lower plot). This strategy will only be successful when both compounds elute differently and have significantly different abundances.
From the fractions of the enrichment step, those containing interesting compounds can be selected. Therefore, CCC has become an important tool in the field of lipid separations.
CCC separation of compounds of the unsaponifiable matter of lipids
Carotenoids
Crude plant extracts with carotenoids usually contain only a few compounds, with carotenoids being particularly nonpolar. For this reason, further enrichment steps can be avoided, and these compounds can be isolated in high purity with one CCC run. Carotenoids were successfully fractionated by using n-heptane/chloroform/acetonitrile (10:3:7) or n-hexane/ethanol/water (4/3/1 or 6/5/1.3) for the isolation of lutein [11,12]. Due to their huge difference in polarity, free xanthophylls and esterified ones are eluting well-separated by CCC.
Sterols
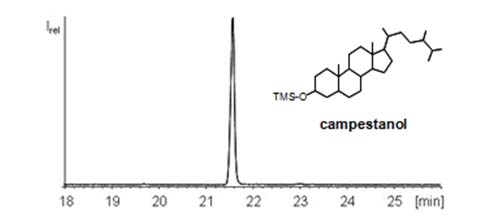
CCC has also been used for the enrichment of β-sitosterol from a phytosterol standard (97% purity) with n-heptane-ethyl acetate-acetonitrile (5:1:5, v/v/v) as the solvent system [13]. The solvent system n-hexane-ethyl acetate-acetonitrile (5:1:5, v/v/v) was used to isolate α-spinasterol (96.7 purity) and β-sitosterol (97.2% purity) from the root of Chinese herb Adenophora tetraphlla [14]. The two-phase solvent system consisting of n-hexane-ethyl acetate-butanol-methanol-water (3.5:0.3:0.5:2.5:0.3, v/v) was used to isolate ergosterol (36.5 mg, purity 92.0%) and stigmasterol (43.6 mg; purity 95.5%) from 100 g of the Chinese herb A. roxburghii [15]. Higher purities (99% or more) usually require two or even three CCC runs (Figure 7).
Figure 7: GC/MS chromatogram of the trimethylsilyl ether of campestanol isolated from vegetable oil by means of CCC [9].
Conclusions
As with other chromatography instrumentation, the development of CCC techniques is a continuing story. Novel aspects focus on the coupling of CCC with mass spectrometry and/or the use of multidimensional CCC [16]. A related CCC technique named pH-zone refining [17] has also been introduced to lipid analysis [18]. Last but not least, the related centrifugal partition chromatography (CPC) has come into fashion. In CPC, the hydrodynamic mode of CCC is substituted with a hydrostatic system. This is obtained by substituting the coils by small elution separation chambers which are connected with each other [16].
References
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A, 1065, 145-168 (2005) (DOI: 10.1016/j.chroma.2004.12.044).
- Ito, Y. and Bowman, R.L. Countercurrent chromatography: Liquid-liquid partition chromatography without solid support. Science, 167, 281-283 (1970).
- Schröder, M. and Vetter, W. High-speed counter-current chromatographic separation of phytosterols. Anal. Bioanal. Chem., 400, 3615-3623 (2011); (DOI: 10.1007/s00216-011-4995-2).
- Hammann, S., Tillmann, U., Schröder, M. and Vetter, W. Profiling the fatty acids from a strain of the microalgae Alexandrium tamarense by means of high-speed counter-current chromatography and gas chromatography coupled with mass spectrometry. J. Chromatogr. A, 1312, 93-103 (2013) (DOI: 10.1016/j.chroma.2013.08.090).
- Englert, M. and Vetter, W. Solvent systems with n-hexane and/or cyclohexane in countercurrent chromatography - Physico-chemical parameters and their impact on the separation of alkyl hydroxybenzoates. J. Chromatogr. A, 1342, 54-62 (2014); (DOI: 10.1016/j.chroma.2014.03.050).
- Miwa, T.K., Mikolajezak, K.L., Earle, F.R. and Wolff, I.A. Gas chromatographic characterization of fatty acids - identification constants for mono- and dicarboxylic methyl esters. Anal. Chem., 32, 1739-1742 (1960).
- Li, D., Schröder, S. and Vetter, W. Isolation of 6,9,12,15-hexadecatetraenoic fatty acid (16:4n-1) methyl ester from transesterified fish oil by HSCCC. Chromatographia, 75, 1-6 (2012) (DOI: 10.1007/s10337-011-2155-7).
- Bousquet, O. and Le Goffic, F. Counter-current chromatographic separation of polyunsaturated fatty acids. J. Chromatogr. A, 704, 211-216 (1995); (DOI: 10.1016/0021-9673(94)01233-5).
- Schröder, M. and Vetter, W. Investigation of unsaponifiable matter of plant oils and isolation of eight phytosterols by means of high-speed counter-current chromatography. J. Chromatogr. A, 1237, 96-105 (2012) (DOI: 10.1016/j.chroma.2012.03.033).
- Schröder, M. and Vetter, W. Detection of 430 fatty acid methyl esters from a transesterified butter sample. J. Amer. Oil Chem. Soc., 90, 771-790 (2013) (DOI: 10.1007/s11746-013-2218-z).
- Li, H.-B., Chen, F., Zhang, T.-Y., Yang, F.-Q. and Xu, G.-Q. Preparative isolation and purification of lutein from the microalga Chlorella vulgaris by high-speed counter-current chromatography. J. Chromatogr. A, 905, 151–155 (2001); (DOI: 10.1016/S0021-9673(00)00987-0).
- Aman, R., Carle, R., Conrad, J., Beifuss, U. and Schieber, A. Isolation of carotenoids from plant materials and dietary supplements by high-speed counter-current chromatography. J. Chromatogr. A, 1074, 99–105 (2005); (DOI: 10.1016/j.chroma.2005.03.055).
- Zhou, Y., Chen, F. and Zongcheng, L. Preparative separation of β-sitosterol by high speed countercurrent chromatography. J. Liq. Chrom. Rel. Technol., 25, 1693-1701 (2002).
- Yao, S., Liu, R., Huang, X. and Kong L. Preparative isolation and purification of chemical constituents from the root of Adenophora tetraphlla by high-speed counter-current chromatography with evaporative light scattering detection. J. Chromatogr. A, 1139, 254-262 (2007) (DOI: 10.1016/j.chroma.2006.11.056)
- Huang, L., Cao, Y., Xu, H. and Chen G. Separation and purification of ergosterol and stigmasterol in Anoectochilus roxburghii (wall) Lindl by high-speed counter-current chromatography. J. Sep. Sci., 34, 385-392 (2011); (DOI: 10.1002/jssc.201000577).
- Michel, T., Destandau, E. and Elfakir, C. New advances in countercurrent chromatography and centrifugal partition chromatography: focus on coupling strategy. Anal. Bioanal. Chem., 406, 957–969 (2014); (DOI: 10.1007/s00216-013-7017-8).
- Ito., Y. and Ma, Y. pH-zone-refining countercurrent chromatography. J. Chromatogr. A, 753, 1-36 (1996); (DOI: 10.1016/S0021-9673(96)00565-1).
- Song, G., Li, X., Du, J. and Wang, J. Preparative separation of conjugated linoleic acids (CLAs) from fermented Camellia oleifera Abel cake by β-cyclodextrin (β-CD) encapsulation using pH-zone-refining countercurrent chromatography. Food Chem., 146, 437-442 (2014) (DOI: 10.1016/j.foodchem.2013.09.097).
September 9, 2014
In This Section
- Solid-phase extraction columns in the analysis of lipids
- Preparation of Ester Derivatives of Fatty Acids for Chromatographic Analysis
- Preparation of Lipid Extracts Tissues
- The Chromatographic Resolution of Chiral Lipids
- Detectors for HPLC of Lipids with Special Reference to Evaporative Lght-Scattering Detection
- Why Doesn't Your Method Work When I Try It?
- Laboratory Accreditation in a Lipid Analysis Context
- What Column do I Need for Gas Chromatographic Analysis of Fatty Acids?
- Fatty Acid Analysis by HPLC
- Alternatives to Methyl Esters for GC Analysis of Fatty Acids
- A Practical Guide to the Analysis of Conjugated Linoleic Acid (CLA)
- Application of Infrared Spectroscopy to the Rapid Determination of Total Saturated, trans, Monounsaturated, and Polyunsaturated Fatty Acids
- The Use of Lithiated Adducts for Structural Analysis of Acylglycerols by Mass Spectrometry with Electrospray Ionization
- Identification of FAME Double Bond Location by Covalent Adduct Chemical Ionization (CACI) Tandem Mass Spectrometry
- The Use of Countercurrent Chromatography (CCC) in Lipid Analysis
- Gas Chromatographic Analysis of Plant Sterols
- Analysis of Tocopherols and Tocotrienols by HPLC
- Reversed-Phase HPLC of Triacylglycerols
- Structural Analysis of Triacylglycerols
- Thin-Layer Chromatography of Lipids
- High-temperature Gas Chromatography of Triacylglycerols
- Modification of an AOCS Official Method for Crude Oil Content in Distillers Grains and Other Agricultural Materials
- Lipidomics - A Personal View