Structural Analysis of Triacylglycerols
The Author: William W. Christie, James Hutton Institute (and Mylnefield Lipid Analysis), Invergowrie, Dundee (DD2 5DA), Scotland.
As nearly all the commercially important fats and oils of animal and plant origin consist almost exclusively of the simple lipid class – triacylglycerols, there has been a considerable impetus to provide methods for structural analysis. These are summarized here.
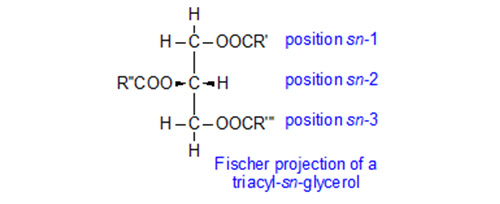 For some years, it has been recognised that the compositions of the fatty acids on the different positions of the glycerol moiety of triacylglycerols can be very different. Position 2 is of course distinctive in that it is a secondary hydroxyl group, but it is not always recognised that the two primary positions are different stereochemically as there is a centre of asymmetry. Triacylglycerols therefore exist in enantiomeric forms. The positions are defined by a 'stereospecific numbering' (sn) system as sn-1, sn-2 and sn-3, and in natural triacyl-sn-glycerols, each can have a distinctive fatty acid composition, as is discussed in greater detail in our web page on triacylglycerols.
For some years, it has been recognised that the compositions of the fatty acids on the different positions of the glycerol moiety of triacylglycerols can be very different. Position 2 is of course distinctive in that it is a secondary hydroxyl group, but it is not always recognised that the two primary positions are different stereochemically as there is a centre of asymmetry. Triacylglycerols therefore exist in enantiomeric forms. The positions are defined by a 'stereospecific numbering' (sn) system as sn-1, sn-2 and sn-3, and in natural triacyl-sn-glycerols, each can have a distinctive fatty acid composition, as is discussed in greater detail in our web page on triacylglycerols.
Pancreatic lipase hydrolysis is a relatively simple method for the determination of the composition of position 2, i.e. for “regiospecific” analysis. This can also be accomplished by some modern mass spectrometry methods and by nuclear magnetic resonance spectroscopy. However, the compositions of positions sn-1 and sn-3 could until recently only be obtained by complex "stereospecific analysis" procedures with many steps involving degradation, synthesis, enzymatic hydrolysis and chromatographic separation of the products. The task has been simplified by the development of methods involving chiral chromatography. These methods have been used to determine the structures of many natural triacyl-sn-glycerols, and some surprisingly asymmetric fats have been found, with first prize going to milk fat in which all the short-chain fatty acids (butyric and hexanoic acids) are concentrated in position sn-3 with none in positions sn-1 and -2.
Regiospecific Analysis of Triacylglycerols by Hydrolysis with Pancreatic Lipase
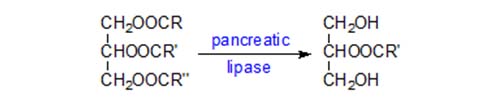
The composition of position sn-2 of triacylglycerols can be determined by incubating them with the enzyme pancreatic lipase in an appropriate buffer. The fatty acids are hydrolysed from the primary positions leaving a 2-monoacyl-sn-glycerol, which can be isolated for determination of its fatty acid composition.
Pig pancreatin, a powder obtained by dehydrating and defatting pig pancreas with acetone and diethyl ether, is the most widely used source of the enzyme; it is stable for long periods of time and is available from suppliers of biochemicals.
All straight-chain saturated fatty acids in the normal chain-length range and most mono-, di- and trienoic acids are apparently hydrolysed from the primary positions of triacylglycerols at about the same rate, although the ester bonds of polyunsaturated fatty acids such as docosahexaenoic (e.g. in fish oils), trans-3-hexadecenoic (e.g. from some plant sources), γ-linolenic acid and phytanic acid to glycerol are hydrolysed more slowly, possibly as a result of steric hindrance caused by the proximity of substituent groups to the ester bonds. In addition, the enzyme hydrolyses triacylglycerol molecules that contain short-chain fatty acids more rapidly than molecules containing only longer-chain fatty acids. For example, with a triacylglycerol such as 1-butyro-2,3-dipalmitin, both the fatty acids on the primary positions were found to be hydrolysed at about the same rate but faster than from tripalmitin. Fortunately, when the enzyme is used with triacylglycerols containing a more normal range of fatty acids, little specificity is observed and the 2-monoacylglycerols produced can be considered in practice to be representative of those in the native triacylglycerols.
Calcium ions are essential and bile salts helpful for the reaction, and it is necessary that the triacylglycerols be well dispersed by vigorous shaking, as they must be in a micellar form for hydrolysis to occur. For this reason, methyl oleate or hexane have sometimes been added to relatively saturated fats, with high melting-points, as carriers; the latter may not always give reliable results, and pre-incubation at 42°C for 5 min has been recommended instead. In structural studies, the concentrations of the various cations, bile salts and the enzyme, the pH of the buffer and the temperature are adjusted to their optima so that an appreciable degree of hydrolysis (50-60% is sufficient) occurs in a short time (1-2 min). Undesirable side reactions such as acyl migration are thereby minimized.
A semimicro method developed by Luddy et al. (J. Am. Oil Chem. Soc., 41, 693-696 (1964)) is recommended as the best practical procedure for the purpose. In essence, a buffer solution (pH 8) containing bile salts and calcium chloride is added to the triacylglycerols at 40°C. After a brief equilibration period, the enzyme preparation is added and the mixture is shaken very vigorously for 1-2 minutes, when about 50% hydrolysis should have occurred. The reaction is then stopped by addition of acid, the lipid products are extracted, and the monoacylglycerols are isolated by thin-layer chromatography for methylation and gas chromatographic analysis.
As hydrolysis may not be completely random and as there may be some contamination from lipids endogenous to the enzyme preparation, or from fatty acids liberated from position sn-2 following acyl migration, the free fatty acids released may be somewhat different from the composition originally present in the primary positions of the triacylglycerols. The mean composition of each fatty acid in positions sn-1 and sn-3 can, however, be calculated from its concentration in the intact triacylglycerol and in position sn-2 by means of the relationship -
[position 1 or 3] = (3 x [triacylglycerol] - [position 2])/2
The mould Rhizopus arrhizus secretes an extracellular lipase that also has an absolute specificity for the primary bonds of glycerolipids and so resembles pancreatic lipase in many respects. It differs in that it does not have an absolute requirement for calcium ions and is not activated by bile salts. Although it offers no appreciable advantages over pancreatic lipase in most instances, there are a few applications where it has been preferred. In addition, some purely chemical methods have been published.
Regiospecific Analysis of Triacylglycerols by Spectroscopic Means
13C-Nuclear magnetic resonance (NMR)spectroscopy can be used to obtain quantitative information on the distribution of fatty acids in position 2 of triacyl-sn-glycerols. It appears to be especially useful for fish oils which are not easily analysed by other means. Amongst other disadvantages, it does not distinguish between different saturated components, or sometimes between unsaturated fatty acids with the first double bond in the same position. The technique is discussed in greater detail in the NMR pages of this web site.
Tandem mass spectrometry methods, especially with electrospray ionization, can also be used to determine the regiospecific distribution of fatty acids within triacylglycerols, but this would take us into the realm of the specialist.
Stereospecific Analysis of Triacyl-sn-glycerols - Methods involving Stereospecific Enzymes
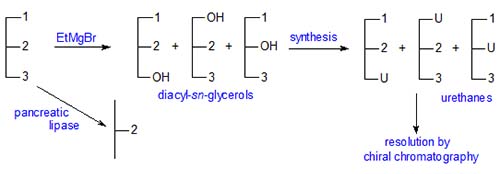
No lipolytic enzyme has yet been isolated that is capable of distinguishing between position 1 and 3 of a triacyl-sn-glycerol. Nonetheless, a number of ingenious stereospecific analysis procedures have been developed for determining the compositions of positions sn-1, sn-2 and sn-3. Several methods have been described over the years, but nowadays there are two main approaches and one making use of specific lipases is described here.
Brockerhoff (J. Lipid Res., 6, 10-15 (1965)) devised the first stereospecific analysis procedure of general applicability. His method has been much modified by subsequent research workers but in essence has stood the test of time. The basic procedure is outlined below. α,β-Diacylglycerols (an equimolar mixture of the 1,2- and 2,3-sn-isomers) are first prepared by partial hydrolysis for conversion synthetically to phospholipid derivatives, which are in turn hydrolysed by the phospholipase A of snake venom, an enzyme which reacts only with the "natural" 1,2-diacyl-sn-glycerophosphatide. The products are a lysophosphatide that contains the fatty acids originally present in position sn-1, free fatty acids released from position sn-2 and the unchanged "unnatural" 2,3-diacyl-sn-phosphatide. After isolation of each product and transesterification, the fatty acid compositions (in mol per cent) are determined by gas chromatography. In addition, the composition of position sn-2 is determined independently by means of pancreatic lipolysis as a check.
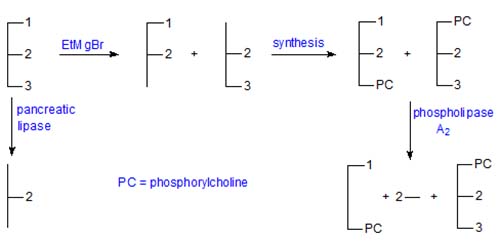
Only the fatty acid composition of position sn-3 is not determined directly by this method, but the amount of each fatty acid in this position can be calculated from the analysis of the original triacylglycerol and those of positions sn-1 and sn-2, or from the analysis of the 2,3-diacyl-sn-phosphatide, i.e. for each component
[position 3] = 3 x [triacylglycerol] - [position sn-1] - [position sn-2]
or [position 3] = 2 x [2,3-diacyl-sn-phosphatide] - [position sn-2]
The key to success with the procedure lies in the preparation of the intermediate α,β-diacylglycerols, which must be generated in a random manner so that the fatty acid compositions of the various positions are identical to those in the original triacylglycerols. There must be no selectivity for specific fatty acids or fatty acid combinations, and the least possible acyl migration during their formation. Hydrolysis with pancreatic lipase was used initially but a Grignard reagent, specifically ethyl magnesium bromide, is now preferred as it has no known fatty acid specificities and causes less acyl migration than other methods tested. In practice, such acyl migration as does occur causes some contamination (6-10%) of the 1,3-diacylglycerols but much less of the α,β-diacylglycerols in which the primary positions are virtually unchanged. I must state that I am not convinced that the problem has been fully solved. The α,β-diacylglycerols required for the procedure must not come in contact with polar solvents or be heated, and they must be isolated immediately by means of TLC on layers of silica gel G impregnated with boric acid for conversion without delay to phosphatides, which can in turn be purified by TLC or other methods.
In the original Brockerhoff procedure, phosphatidylphenols were prepared from the diacylglycerols, but it was later shown that it is relatively easy to prepare phosphatidylcholines, the chromatographic properties of which are better understood. They can also be hydrolysed with some stereoselectivity with phospholipase C, offering an additional analytical option.
Full experimental details of a recommended procedure, which incorporates features developed in a number of laboratories, have been published (Christie, 1986; see reading list below). Although the procedure is rather complex and involves a number of steps, it is capable of reasonable precision in the hands of skilled workers. Analyses should not be accepted unless the results for major components in positions sn-2 and sn-3, determined by both available methods, agree within 4% (absolute).
The main drawback to this approach to the stereospecific analysis of triacyl-sn-glycerols is that the fatty acid composition of position sn-3 is not determined directly, so that the proportionate errors in the results for minor fatty acids in this position can be considerable. Small negative values are sometimes obtained and small positive values may arise in calculations for a component that is not present in the position at all. This could no doubt be overcome by reacting the unchanged 2,3-diacyl-sn-phosphatide with the lipase of Rhizopus arrhizus to release the fatty acids from position sn-3 for direct analysis.
Stereospecific Analysis of Triacyl-sn-glycerols - Methods involving Chiral Chromatography
In the last few years, alternative approaches to stereospecific analysis have been described that use simple chemical degradative and derivatization steps and the methodology of chiral chromatography. One such method was developed in our laboratory. The principle is dependent on the fact that diastereomeric compounds can be resolved by adsorption chromatography on silica gel, because they have different physical and chemical properties. Thus a chiral derivatizing agent (R)-X will react with a racemic substance (R,S)-Y to form diastereomers as follows.
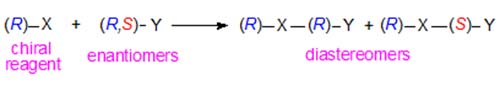
The degree of separation of the two diastereomers in a chromatographic system will depend on the chiral structures of X and Y and the manner of their interactions with the mobile and stationary phases. In addition, it is noteworthy that the order of elution of the diastereomeric derivatives is reversed if the other enantiomer of the reagent, i.e. (S)-X, can be employed. (S)-(+)-1-(1-Naphthyl)ethyl urethane derivatives of diacyl-sn-glycerols are preferred for the purpose. With the HPLC column of silica gel used in this work, it appears that the presence of the hydrogen atom on the nitrogen between the chiral centres is essential to the separation process; it is presumed to be a primary site for hydrogen bonding to silanols on the adsorbent surface.
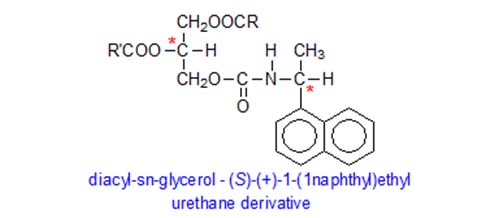 The first step in the stereospecific analysis procedure is identical to that in most other methods, i.e. partial hydrolysis of the triglycerides with ethyl magnesium bromide to a mixture of sn-1,2-, 2,3- and 1,3-diacylglycerols, amongst other products. Next, the products are reacted with a chiral derivatizing agent, (S)-(+)-1-(1-naphthyl)ethyl isocyanate and the diacyl-sn-glycerol urethane derivatives are isolated by chromatography on solid-phase extraction columns containing an octadecylsilyl phase; by-products of low molecular-weight and excess derivatizing reagents are eluted first and discarded, before the required diacylglycerol urethane derivatives are recovered.
The first step in the stereospecific analysis procedure is identical to that in most other methods, i.e. partial hydrolysis of the triglycerides with ethyl magnesium bromide to a mixture of sn-1,2-, 2,3- and 1,3-diacylglycerols, amongst other products. Next, the products are reacted with a chiral derivatizing agent, (S)-(+)-1-(1-naphthyl)ethyl isocyanate and the diacyl-sn-glycerol urethane derivatives are isolated by chromatography on solid-phase extraction columns containing an octadecylsilyl phase; by-products of low molecular-weight and excess derivatizing reagents are eluted first and discarded, before the required diacylglycerol urethane derivatives are recovered.
The most important step involves resolution of the diacylglycerol urethanes by HPLC on a column of silica gel. A simple isocratic mobile phase is employed, and since the derivatives absorb strongly in the UV spectrum, detection is straightforward. 1,3-Diacylglycerol urethanes elute early and this fraction is easily recovered; however, as they are more susceptible to acyl migration than the other products of interest, they are not used further. As the derivatizing agent is chiral and a single enantiomer, the 1,2- and 2,3-diacyl-sn-glycerol urethanes formed from it are now diastereomers, so they are separable in a non-chiral environment.
In practice, the 1,2-diacyl-sn-glycerol derivatives elute ahead of the 2,3-diastereomers, and the two distinct fractions can be collected. Some separation of molecular species also occurs within each diastereomeric fraction, and while this might be considered an advantage in some circumstances, it is here something of a nuisance as it restricts the range of fatty acid components in the triacylglycerols that can be investigated. Fortunately, most of the common fats with C16 and C18 fatty acids are in the practical range, and it may be possible with further development to extend this eventually.
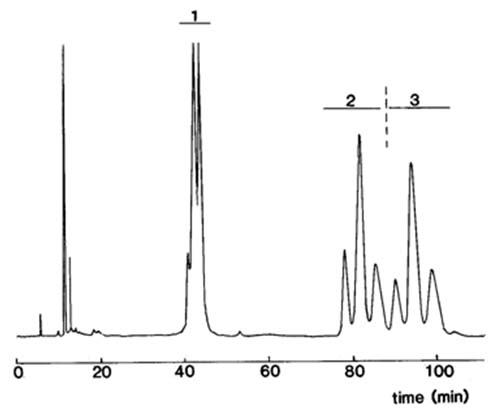
The final step involves methylation of each of the fractions for analysis by GC with the highest precision possible, when the results for the positional distributions are simply a matter for calculation. For example, as the fatty acid composition of the intact triacylglycerols is known, and that of the 1,2-diacyl-sn-glycerol derivatives has been determined, it is simple arithmetic to calculate the composition of position sn-3. Similarly, the composition of position sn-1 can be calculated once that of the 2,3-diacyl-sn-glycerols is known. That of position sn-2 is calculated by difference (or by an independent hydrolysis with pancreatic lipase). Thus, the composition of all three positions is determined, without resort to enzymes, by using standard chromatography columns and derivatizing agents that are available from commercial sources. The separation of diacyl-sn-glycerol urethane derivatives from palm oil by chiral HPLC is illustrated next (Fig. 1).
Figure 1. Separation of (S)-(+)-1-(1-naphthyl)ethyl urethane derivatives of diacyl-sn-glycerols (DG) from palm oil by HPLC on two columns of silica gel (HypersilTM 3μ, 250 × 4.6 mm) with isooctane with 0.3% isopropanol (containing 2% water) as mobile phase (Christie et al., 1991). Abbreviations: 1 = 1,3-DG, 2 = 1,2-DG, 3 = 2,3-DG. Reproduced by kind permission of the Journal of the American Oil Chemists’ Society.
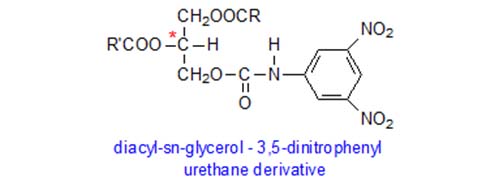 Professor Takagi (1991) and colleagues in Japan adopted a related but distinct procedure. They converted mono- and diacyl-sn-glycerols prepared from triacylglycerols to the 3,5-dinitrophenyl urethane (DNPU) derivatives for resolution by HPLC on columns containing a stationary phase with chiral moieties bonded chemically to a base of silica gel (e.g. SumipaxTM OA-4100). The 3,5-dinitrophenyl moieties of the urethanes contribute to charge-transfer interactions with functional groups having pi electrons on the stationary phase and thus aid resolution.
Professor Takagi (1991) and colleagues in Japan adopted a related but distinct procedure. They converted mono- and diacyl-sn-glycerols prepared from triacylglycerols to the 3,5-dinitrophenyl urethane (DNPU) derivatives for resolution by HPLC on columns containing a stationary phase with chiral moieties bonded chemically to a base of silica gel (e.g. SumipaxTM OA-4100). The 3,5-dinitrophenyl moieties of the urethanes contribute to charge-transfer interactions with functional groups having pi electrons on the stationary phase and thus aid resolution.
Although diacylglycerols could be used in a manner analogous to the above, 1-, 2- and 3-monoacyl-sn-glycerols are prepared from triacylglycerols in their preferred procedure by partial hydrolysis with ethyl magnesium bromide. The 1(3)-forms are separated from the 2-isomers by TLC on silica gel impregnated with boric acid, before being converted to the DNPU derivatives. Then, the 1- and 3-monoacyl-sn-glycerol derivatives are resolved on a chiral HPLC column. After methylation and GC analysis, the distributions of fatty acids in each of positions sn-1, sn-2 and sn-3 can be calculated from the data. By means of lowering the column temperature and slowing down the flow-rate, the method can even be applied to such complex triacyl-sn-glycerols as fish oils.
This last method has the virtue of enabling direct determination of the composition of all three positions, but I would be concerned that the monoacylglycerols were generated from triacylglycerols by the action of the Grignard reagent in a truly random manner, although this is claimed to be the case.
Molecular Species Composition of Triacylglycerols
It is well known that natural triacylglycerols exist in the form of a large number of distinct molecular species. The problem can be extremely complicated; for example, a triacylglycerol with only five different fatty acid constituents may consist of 75 different molecular species not including enantiomers, or 125 if enantiomers are included. With milk fat, the number of different molecular species reaches astronomical proportions. A vast amount of data is available in the literature, and it is not possible to summarize it here. Indeed to my knowledge, there does not appear to be any substantial review on the topic of molecular species compositions anywhere. This document contains a brief note only on the methods, but the topic is treated in other web pages on this site.
Three basic methods of analysis are available – silver ion chromatography, reversed-phase chromatography and high-temperature gas chromatography. In silver ion chromatography, separation is in essence according to the degree of unsaturation of the triacylglycerol molecules, while in reversed-phase chromatography separation is both by the combined degree of unsaturation and by chain lengths of the fatty acid moieties. Originally, thin-layer techniques only were available, but nowadays most analysts resort to high-performance liquid chromatography. While some excellent separations of triacylglycerols by gas chromatography have been published, the technology is close to its absolute limits, and quantification presents difficulties that are not always recognized. Separation can be by chain length only or can include degree of unsaturation with appropriate column types. These are important topics, which are best discussed in greater detail elsewhere on this site. Silver ion chromatography followed by reversed-phase chromatography of the fractions is an especially powerful approach to analysis of molecular species.
Reversed-phase chromatography of triacylglycerols is discussed in this section of this web site, or you can consult my recent book cited below. Similarly, gas chromatographic analysis is discussed in 'GC and Lipids. Chapter 8' and there is a separate cautionary note. Other chromatographic procedures are discussed in Chapter 9 of the book. Nowadays, tandem mass spectrometry is being used increasingly for the purpose with atmospheric-pressure chemical ionization or electrospray ionization being favoured. These techniques can be used in conjunction with liquid chromatography or on the intact lipid, and give information both on molecular species compositions and on regiospecific (but not stereospecific) distributions of fatty acids in triacyl-sn-glycerols
Molecular Species Composition of Triacylglycerols and Molecular Distribution Theories
There have been innumerable attempts to relate the composition of the molecular species of natural triacylglycerols to their overall fatty acid composition or to the compositions of the various positions of the glycerol moiety. For example, a triacylglycerol containing 50% fatty acid A and 50% fatty acid B could contain only two molecular species that could be designated AAA and BBB, or other species might exist of the form AAB, ABA, etc. In natural triacylglycerols, the proportions of each molecular species depend on the specificities of the enzymes (acyl transferases) involved in their biosynthesis and on the pool of fatty acids available. In addition, some remodelling can occur following synthesis. Simple mathematical models, based on the suggested specificities of these enzymes, have been devised that utilize regio- and stereospecific analysis data to calculate the proportions of individual molecular species, for testing against the appropriate analyses.
However, it should be recognized that many of the early models proposed had little or no biochemical foundation. This is not to denigrate the work of the early pioneers in the field, who were making entirely worthy attempts, with data of limited accuracy, to find methods of practical value for calculating the main molecular fractions controlling the physical properties of natural fats. When attempts were made to modernize the theory by the application of the results of stereospecific analyses, no consistent patterns emerged. In my opinion, molecular distribution theories are best forgotten
Recommended Reading
- Buchgraber, M., Ulberth, F., Emons, H. and Anklam, E. Triacylglycerol profiling by using chromatographic techniques. Eur. J. Lipid Sci. Technol., 106, 621-648 (2004) (DOI: 10.1002/ejlt.200400986).
- Byrdwell, W.C. Qualitative and quantitative analysis of triacylglycerols by atmospheric pressure ionization (APCI and ESI) mass spectrometry techniques. In: Modern Methods for Lipid Analysis by Liquid Chromatography/Mass Spectrometry and Related Techniques, pp. 298-412 (edited by W.C. Byrdwell, AOCS Press, Champaign) (2005).
- Christie, W.W. The positional distribution of fatty acids in triglycerides. In: Analysis of Oils and Fats, pp. 313-339 (ed. R.J. Hamilton and J.B. Rossell, Elsevier Applied Science, London) (1986).
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Bridgwater, U.K.) (2010) - http://store.elsevier.com/product.jsp?locale=en_US&isbn=9780955251245.
- Christie, W.W., Nikolova-Damyanova, B., Laakso, P. and Herslof, B. Stereospecific analysis of triacyl-sn-glycerols via resolution of diastereomeric diacylglycerol derivatives by high-performance liquid chromatography on silica. J. Am. Oil Chem. Soc., 68, 695-701 (1991).
- Kuksis, A. Analysis of positional isomers of glycerolipids by non-enzymatic methods. In: Advances in Lipid Research – Three, pp. 1-36 (edited by W.W. Christie, Oily Press, Dundee) (1996).
- Kuksis, A. and Itabashi, Y. Regio- and stereospecific analysis of glycerolipids. Methods, 36, 172-185 (2005) (DOI: 10.1016/j.ymeth.2004.11.001).
- Laakso, P. Analysis of triacylglycerols: approaching the molecular composition of natural mixtures. Food Rev. Int., 12, 199-250 (1996).
- Takagi, T. Chromatographic resolution of chiral lipid derivatives. Prog. Lipid Res., 29, 277-298 (1991).
In This Section
- Solid-phase extraction columns in the analysis of lipids
- Preparation of Ester Derivatives of Fatty Acids for Chromatographic Analysis
- Preparation of Lipid Extracts Tissues
- The Chromatographic Resolution of Chiral Lipids
- Detectors for HPLC of Lipids with Special Reference to Evaporative Lght-Scattering Detection
- Why Doesn't Your Method Work When I Try It?
- Laboratory Accreditation in a Lipid Analysis Context
- What Column do I Need for Gas Chromatographic Analysis of Fatty Acids?
- Fatty Acid Analysis by HPLC
- Alternatives to Methyl Esters for GC Analysis of Fatty Acids
- A Practical Guide to the Analysis of Conjugated Linoleic Acid (CLA)
- Application of Infrared Spectroscopy to the Rapid Determination of Total Saturated, trans, Monounsaturated, and Polyunsaturated Fatty Acids
- The Use of Lithiated Adducts for Structural Analysis of Acylglycerols by Mass Spectrometry with Electrospray Ionization
- Identification of FAME Double Bond Location by Covalent Adduct Chemical Ionization (CACI) Tandem Mass Spectrometry
- The Use of Countercurrent Chromatography (CCC) in Lipid Analysis
- Gas Chromatographic Analysis of Plant Sterols
- Analysis of Tocopherols and Tocotrienols by HPLC
- Reversed-Phase HPLC of Triacylglycerols
- Structural Analysis of Triacylglycerols
- Thin-Layer Chromatography of Lipids
- High-temperature Gas Chromatography of Triacylglycerols
- Modification of an AOCS Official Method for Crude Oil Content in Distillers Grains and Other Agricultural Materials
- Lipidomics - A Personal View