Esters of Glycerol and Other Polyhydric Alcohols
The Author: Frank D. Gunstone, James Hutton Institute (and Mylnefield Lipid Analysis), Invergowrie, Dundee (DD2 5DA), Scotland
Monoacylglycerols (MAG) and diacylglycerols (DAG) are important emulsifying agents. They are also used in acetylated forms. Acylated derivatives of other polyhydric alcohols such as carbohydrates, propane-1,2-diol, trimethylolpropane, and pentaerythritol are also useful compounds. Chemical shifts for many of these compounds have been reported.
- There are five different classes of glycerol esters (see Table 1), and chemical shifts associated with the glycerol carbon are useful for both qualitative and quantitative purposes, especially the glyc-2 signals. Values for glyc-2 lie between 68 and 75 ppm and those for glyc-1 and 3 are between 61 and 66 ppm. In "symmetrical" molecules (2-MAG, 1,3-DAG, and TAG) the signals for glyc-1 and glyc-3 are the same. They differ in the "unsymmetrical" molecules. These chemical shifts can be used to analyse mixtures of glycerol esters (Gunstone, 1991, 1993) and the results of a ring test have been reported (Grone et al., 1998). Signals for C1 and C2 in the acyl chains also differ between the various glycerol esters, but these are less useful (Gunstone, 1991, 1993).
- Chemical shifts for acetylated monoacylglycerols are listed in Table 2.
- Chemical shifts for acylated derivatives of propane-1,2-diol are given in Table 3.
- Chemical shifts for fully acylated derivatives of trimethylolpropane, and pentaerythritol are given in Table 4.
- Sugar esters: chemical shifts are recorded for sucrose-6'-O-mono esters (10:0, 12:0, 14:0, 16:0, and 18:0) and sucrose-6-O-mono esters (12:0, 14:0, 16:0, and 18:0). Full details are given in the original paper (Sarney et al., 1996).
| Table 1. Chemical shifts (ppm) for glycerol carbon atoms in monoacylglycerols (mag), diacylglycerols (dag), and triacylglycerols (tag) based on palmitic and oleic acids (Vlahov. 1996) | ||||||
| Palmitic | Oleic | |||||
| Glyc-1 | Glyc-2 | Glyc-3 | Glyc-1 | Glyc-2 | Glyc-3 | |
| 1-mag | 63.34 | 70.26 | 65.11 | 63.35 | 70.26 | 65.13 |
| 2-mag* | 61.9 | 74.9 | 61.9 | |||
| 1,2-dag | 61.54 | 72.11 | 62.02 | 61.56 | 72.12 | 62.00 |
| 1,3-dag | 65.04 | 68.38 | 65.04 | 65.03 | 68.34 | 65.03 |
| tag | 62.10 | 68.87 | 62.10 | 62.08 | 68.87 | 62.08 |
* Values taken from Gunstone (1991, 1993), fatty acid not indicated. |
||||||
| Table 2. Chemical shifts (ppm) for glycerol and other carbon atoms in acetylated monoacylglycerols (Gunstone, 1991). Compounds 1-4 are the 1-monoacylglycerol (1), its 2-acetate (2), 3-acetate (3), and 2,3-diacetate (4) | ||||
| 1 | 2 | 3 | 4 | |
| Glyc-1 | 65.15 | 62.07 | 65.00 | 62.00 |
| Glyc-2 | 70.26 | 72.39 | 68.14 | 69.16 |
| Glyc-3 | 63.39 | 61.40 | 65.26 | 62.33 |
| C1 | 174.31 | 173.84 | 173.96 | 173.38 |
| COCH3 (α) | - | - | 20.79 | 20.69 |
| 171.10 | 170.57 | |||
| COCH3 (β) | - | 21.00 | - | 20.88 |
| 170.98 | 170.16 | |||
| Table 3. Chemical shifts (ppm) for mono and di-esters of propane-1,2-diol (Gunstone, 1993) | ||||||
| C1 | C2 | C3 | P1 | P2 | P3 | |
| 1-ester | 173.99 | 34.23 | 24.99 | 69.46 | 66.13 | 19.2 |
| 2-ester | 173.99 | 34.58 | 25.04 | 65.92 | 71.77 | 16.25 |
| 1,2-diester | 173.51 | 34.51 | 25.03 | 65.42 | 67.98 | 16.5 |
| 173.24 | 34.19 | 24.97 | ||||
| Table 4. Chemical shifts (ppm) for fully acylated derivarives of trimethylolpropane (TMP), and pentaerythritol (PE) (Black and Gunstone, 1990). | ||||||||
| a | b | c | d | C1 | y | z | C1 | |
| C2 | 41.98 | 62.43 | 170.25 | |||||
| C6 | 42.24 | 62.28 | 172.79 | |||||
| C7 | 42.12 | 62.23 | 172.99 | |||||
| C8 | 7.48 | 23.36 | 41.01 | 63.72 | 172.71 | 42.17 | 62.26 | 172.95 |
| C9 | 7.47 | 23.38 | 40.92 | 63.76 | 172.73 | 42.25 | 62.27 | 172.81 |
| C10 | 7.42 | 23.31 | 40.89 | 63.75 | 172.96 | 42.18 | 62.27 | 172.88 |
| C12 | 7.39 | 23.33 | 40.87 | 63.82 | 173.26 | 42.11 | 62.28 | 173.11 |
| C7 ether | 7.77 | 23.29 | 43.22 | 71.49 | 71.28 | |||
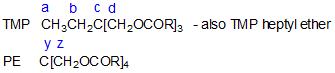 |
||||||||
References
- Black, K.D. and Gunstone, F.D. The synthesis and spectroscopic properties of some polyol esters and ethers. Chem. Phys. Lipids, 56, 169-173 (1990).
- Grone, D., Metternich, H.J. and Schoenfelder, W. Quantitative NMR-spectroscopic analysis of the compounds of glycerol esters of fatty acids. Fett/Lipid, 100, 64-69 (1998).
- Gunstone, F.D. 13C-NMR studies of mono-,di- and triacylglycerols leading to qualitative and semiquantitative information about mixtures of these glycerol esters. Chem. Phys. Lipids, 58, 219-224 (1991).
- Gunstone, F.D. High resolution of 13C-NMR spectroscopy of lipids. In: Advances in Lipid Methodology - Two, pp. 1-68 (ed. W.W. Christie, Oily Press, Dundee) (1993).
- Sarney, D.B., Barnard, M.J., MacManus, D.A. and Vulfson, E.N. Applications of lipases to the regioselective synthesis of sucrose fatty acid monoesters. J. Am. Oil Chem. Soc., 73, 1481-1487 (1996).
- Vlahov, G. Improved quantitative 13C nuclear magnetic resonance criteria for determination of grades of virgin olive oils. The normal ranges of diglycerides in olive oils. J. Am. Oil Chem. Soc., 73, 1201-1203 (1996).
In This Section
- Introduction of NMR
- Saturated Fatty Acids and Methyl Esters
- Alkyl Esters Other than Methyl
- Glycerol Esters
- Non-Conjugated Double Bonds
- Conjugated Linoleic Acid (CLA)
- Acetylenic Fatty Acids and Derivatives
- Branched-Chain and Cyclic Fatty Acids
- Epoxy Fatty Acids
- Hydroxy and Hydroperoxy Fatty Acids
- Oxo Fatty Acids
- Fatty Alcohols
- Some Miscellaneous Fatty Acids
- Quantification by 1H-NMR
- The NMR Spectrum
- Alkanoic Acids
- Monoenoic Acids
- Polyunsaturated Fatty Acids
- Non-Methylene-Interrupted Polyenoic Fatty Acids
- Acids with conjugated unsaturation
- Acetylenic and Allenic Acids and Esters
- Branched-Chain and Cyclic Fatty Acids
- Cyclic Fatty Acids
- Epoxides and Acyclic Ethers
- Hydroxy and Hydroperoxy Acids
- Oxo (Keto) Acids
- Acids, Esters (Alkyl, Glycerol, Waxes), Alcohols and Acetates, Amides, and Nitriles
- Esters of Glycerol and Other Polyhydric Alcohols
- Oils and Fats
- Regiospecific Analysis of Triacylglycerols
