Comprehensive Two Dimensional Gas Chromotography (GC x GC) for Lipid Analysis
The Author: Prof. Giorgia Purcaro, Research and Development Director Chromaleont srl. Messina, Italy
Introduction
One of the main challenges for analysts, in general, is to obtain as much information as possible in the shortest time, while unravelling the chemical profiles of complex samples, such as lipids. Gas chromatography (GC) has always played an important role among analytical techniques. GC was invented approximately 60 years ago and since then, considerable progress has been made [1]. The most important steps toward the increase of separation power in the GC field have been: i) the replacement of the packed column with the more efficient capillary one in 1958 by Golay [2], enabling an approximate ten-time increase in separation power; ii) the introduction of comprehensive two-dimensional GC (GC×GC) in 1991 by Liu and Philips [3].
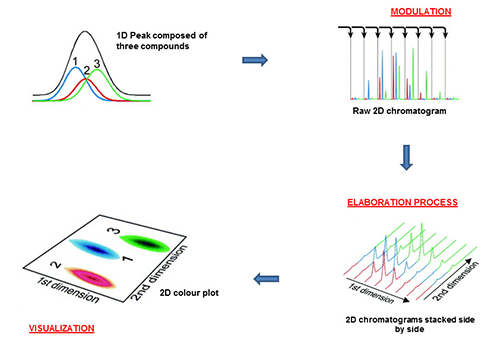
In GC×GC experiments, the separation process is carried out on two columns of different selectivity. An interface, defined as a modulator, is located between the two capillaries. Currently, GC×GC experiments are nearly always performed by using either cryogenic (mainly) or flow modulators. A comprehensive GC separation is obtained when all the components from the first dimension (1D), usually a conventional column (e.g., 25-30 m × 0.25 mm ID × 0.25 µm) are re-analysed on the second dimension (2D), commonly a short micro-bore capillary segment (e.g., 1-2 m × 0.10 mm ID × 0.10 µm). The basic idea is to subject chromatography bands, potentially containing overlapping compounds, to an additional polarity-based separation, in a continuous and sequential manner. A GC×GC system generates a separation peak capacity which is roughly equal to the product of the two peak capacities (peak capacity can be defined as “the number of peaks that can be potentially located in the one or two dimensional space, with a specific resolution value”), in each dimension. Finally, to complete the GC×GC process, data processing and visualization are required. Although basic information on the chromatographic performance can be deduced by expert operators from the modulated raw signal, a transformation process is necessary to visualize and elaborate the results. Dedicated software packages stack second-dimension chromatograms side by side, and considering the modulation time, derive the first and second dimension retention times for each peak. The schematic process from the raw data to the final 2D plot is shown in Figure 1 [4].
Figure 1. Scheme of the generation and visualization process. Reproduced from [4]).
The introduction of GC×GC has not only enabled a better understanding of complex matrices but has also opened a new insight into so-called “well-known” samples, such as lipid samples. The main advantages of comprehensive GC over conventional monodimensional GC, can be summed up in five points, which can be fully or partially exploited depending on the specific application: I) selectivity: since two stationary phases are used; II) separation power: increased resolving power, roughly equal to the product of the two column peak capacities; III) sensitivity: thanks to the re-concentration step during modulation; IV) structured chromatograms: formation of chemically-similar compound patterns when homologous series of compounds are present.
Before describing in more detail the GC×GC field, a brief summary of the most employed terminology in the field is reported in Table 1 for clarification purposes.
| Table 1. Main nomenclature in the GC×GC field. | |
| Term | Definition |
| First dimension (1D) | Symbol for first dimension parameters |
| Second dimension (2D) | Symbol for second dimension parameters |
| Modulation | Process which allows transfer of the chromatographic bands between the two dimensions by fractionation and reinjection of 1D peaks into the 2D column, using an interface called a modulator |
| Modulation period | Time required to perform the entire modulation process |
| Modulation number | The number of modulations for a given first-dimension peak. |
| Cryogenic modulation | Use of a cryogenic fluid (CO2 or N2) for modulation |
| Flow modulation | Use of differential flows for modulation |
| Reinjection | Injection of the chromatographic band deriving from 1D into the 2D column |
| Focusing | Compression of the chromatographic band |
| Single-stage modulation | Accumulation and focusing during a one-step process at one location in the modulator. |
| Dual-stage modulation | Accumulation and focusing during two successive series of processes at two locations in the modulator. |
| Wrap-around | The occurrence of second dimension peaks with retention times that exceed the modulation time. |
GC×GC instrument description
GC×GC systems can be built using the same equipment employed for conventional 1D GC, with only the addition of a modulator as the interface, and using detectors with high acquisition rates, negligible internal volumes, and rapid rise times to accurately re-construct the narrow chromatography bands generated. Samples are introduced by an injector onto the first conventional capillary column, where the first separation occurs; the eluate is then fractionated and re-injected, through the modulator, onto a second capillary column, coated with a different stationary phase, for further separation. The two columns can be located in a single or in two different ovens, the latter option providing a higher degree of flexibility during method optimization. The modulator, which is the heart of the GC×GC system, enables the isolation, re-concentration, and re-injection of chromatography bands from a 1D to a 2D column, in a continuous and sequential manner. The time required to complete a single cycle of events is called a modulation period. The latter parameter is very important since it must be sufficiently low to maintain the resolution achieved on the primary column, but high enough to avoid a loss of sensitivity and to enable the complete elution of the compounds from the second column, before the following injection to avoid wrap-around. At the first stages of development of the technique, wrap-around was considered a serious problem; nowadays it is considered a problem only if co-elutions arise with constituents of the following fraction. In fact, current softwares allow one to correct the 2D elution time by applying a simple time subtraction, thus solving any problems related to the loss of structure in the chromatogram. In 1998, Murphy et al. [5] studied the modulation period in detail, concluding that the best compromise between preservation of the first-dimension resolution and sensitivity can be obtained if every eluting peak is sampled 3 or 4 times. Therefore, in most GC×GC experiments the operational conditions enable the attainment of 15-20 sec 1D peak widths, and the application of 4-6-sec modulation periods.
Considering the modulator device, different approaches have been developed over the years. Here only the most important ones are described. For a thorough discussion on this topic the reader can refer to a recent review [6]. The very first modulator consisted of a 15-cm segment of a thick-film capillary column, divided equally into two-stages, painted with an electrically-conductive film and looped outside the oven, under room temperature conditions [3]. The eluate coming from the 1D column was focalized by the thick stationary-phase film in the first part of the capillary segment. The analyte band was transferred to the second part of the capillary by the application of a 20-msec electrical pulse directed to the first segment of the modulator. At the end of the electrical pulse, the segment cooled down to the initial trapping temperature and the electrical pulse was applied to the second segment of the modulator to reinject the eluate onto the 2D column. Although outstanding results were obtained using this first modulator, the device was fragile and presented low repeatability. Several other heat-based modulators have been developed since the introduction of GC×GC, but this kind of approach was almost completely replaced by cryogenic and, more recently, flow modulators. The first cryogenic modulator was the so-called longitudinally modulated cryogenic system (LMCS), developed by Kinghorn and Marriott [7]. The LMCS consisted of a small CO2 cryogenic trap, characterized by a hollow-sleeve configuration, which moved up and down across a segment of column located at the head of the second dimension. The 1D eluate was trapped and focused by the liquid CO2-cooled trap; then the trap was moved along the capillary column, exposing the previously-cooled spot to the GC oven heat, remobilizing the entrapped compounds onto the second column.
The first commercialized modulator was developed by Ledford and co-workers [8]. It consisted of two perpendicular jets, one cold and one hot, and a delay loop, which enabled the performance of dual-stage modulation. The cold-jet (nitrogen cooled in a bath of liquid nitrogen) works continuously, trapping the primary column effluent by cooling two different segments of the trapping capillary. The perpendicular hot jet is periodically activated, thus deviating the cold jet and warming up the previously cooled capillary segments, assuring effective remobilization of the entrapped compounds and their reinjection onto the 2D column (Figure 2).
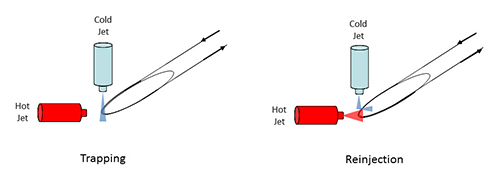
Figure 2. Scheme of a dual-stage loop-type modulator.
Cryogenic modulators perform perfectly in many applications, but the high operation costs cannot be underestimated. Therefore, the development of cryogenic-free modulators has always been highly desirable, considering the high costs of cooling gases. The most interesting alternative was proposed by Bueno and Seeley in 2004 [9], who proposed an easy differential-flow device composed of two sample loops connected through four T-unions. The system was later modified in a simple in-line fluidic modulator, consisting of a single collection channel constructed of deactivated fused silica (Figure 3) [10]. The collection capillary segment was connected to an auxiliary flow through two T-unions, and linked on one side to the primary column outlet and on the other side to the secondary column inlet. The high auxiliary flow was directed in an alternate mode onto the two branches, by activation of a solenoid valve, generating pulsed injections of the chromatography band collected in the accumulation channel.
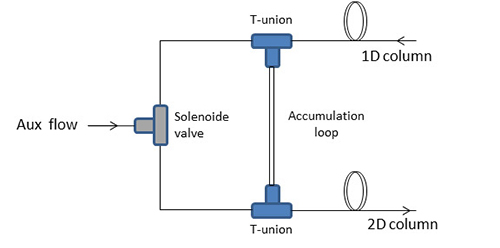
Figure 3. Scheme of the in-line fluidic modulator.
The main drawback of such a system was widely accepted to be the necessity to apply a rather high auxiliary flow rate (20 mL/min) to flush the content of the loop onto the head of the 2D column. Such a high flow rate was not compatible with the pumping capacity of the mass spectrometer (MS) system, thus limiting the first applications of such a device to the coupling with a flame ionization detector (FID). The first attempts to couple a flow modulator with an MS detector were made by diverting the majority of the gas flow to waste, exiting the modulator. However, more recently, it has been proven that the use of such a high flow rate is not necessary and that 6-8 mL are more than enough to perform a satisfactory modulation [11]. The use of a lower flow rate is beneficial not only for the creation of more suitable analytical conditions to perform the 2D separation, but also it greatly reduces the problems related to MS system coupling. Finally, the detector requirements (negligible internal volumes, rapid rise times, fast acquisition rates) to avoid impairing the enhanced resolution obtained with GC×GC are not considered stringent for modern instruments.
GC×GC applications in lipid analysis
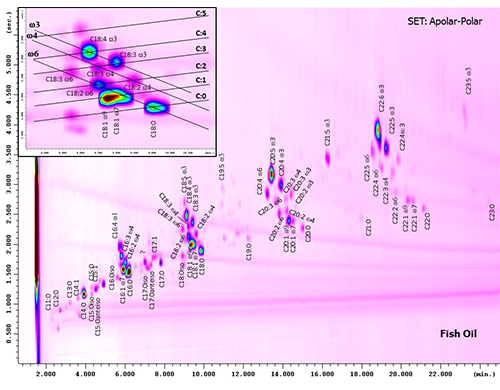
Recently, an increasing number of GC×GC papers dealing with the characterization of the lipid fraction has been published, mainly due to the particular focus on the role of lipids in many fields, such as biological, food, and petrochemical analyses, where the lipid profile plays an important role in understanding many natural, pathological, and degradation processes, as well as the quality of different products. The use of this powerful technique not only has provided a greater understanding of complex samples, but it has also provided a novel insight into well-known samples. The most explicative case-study to fully understand the potentiality of this powerful technique in the lipid field is the analysis of fatty acids (FAs). Conventional GC, with polyethylene glycol-type stationary phases, has been widely employed to separate the most important FAs. However, to fully resolve geometrical and conjugated FA isomers, rather long (up to 100 m) and high-polarity columns are required, such as the bis-cyanopropyl polysiloxane phase or the novel ionic-liquid phases. The application of GC×GC and the formation of chemical-class patterns have been of great support in unravelling the complexity of such samples. In fact, the identification of FAs, in the form of methyl esters (FAMEs), using only MS data and linear retention indices (LRIs), although of great support, are not sufficient enough due to the very similar mass spectra of the isomers, which are instead located in specific positions in the 2D plot, on the basis of the number of carbons and double bonds [12]. A 2D plot obtained from the analysis of fish oil is shown in Figure 4. FAMEs are located in a grid on the basis of their chemical characteristics (highlighted in the expansion-box of Figure 4). Specifically, using an apolar×polar set, saturated compounds are aligned in the lower part of the chromatogram, while FAME retention times in the second dimension increase with the number of double bonds. FAME positions are also dependent on the DB position: compounds with the same ω position are aligned in parallel lines, with the higher ω values eluting before the lower ones (e.g., ω6 FAMEs elute before ω3 ones).
Figure 4. GC×GC-FID chromatogram of fish oil FAMEs obtained using an apolar×polar set. Modified from [12].
Even though the structured nature of the resulting chromatograms does reduce the need for MS, its presence is obviously useful. In FAME experiments, the GC×GC advantages exploited are mainly the increased sensitivity and the formation of group-type patterns.
Over the last few years, new stationary phases have been considered to better separate FAME isomers, among these, the most studied family of stationary phases are ionic liquids (IL), which exhibit both high polarity and thermal stability, and their customizable molecular properties allowing them to be tuned for specific separation requirements. Many IL phases possess a “dual-like” selectivity, which make them suitable for the analysis of both polar and non-polar compounds. Using this kind of stationary phase, a very interesting alternative approach was proposed, where, instead of exploiting different separation mechanisms, the chemical nature of the analyzed compounds was altered by performing an on-line chemical reduction between the first and the second dimensions [13]. Unsaturated FAMEs were reduced to their fully saturated forms after passing through a capillary tube of 50 cm coated with Pd in the presence of H2 as the carrier gas, while the same highly polar stationary phase (SLB-IL111) was used in both dimensions. The hydrogenation of double bonds decreased the dimensionality of the sample solely to chain length, or to the chain length and the position of the methyl group substituent, if iso- and anteiso-FAMEs are also considered. The 1D column generated a complex peak distribution, while the 2D one provided a highly-ordered separation based only on chain length. The proposed method was applied to the analysis of menhaden oil and human colon adenocarcinoma cell FAMEs (the latter result is shown in Figure 5).
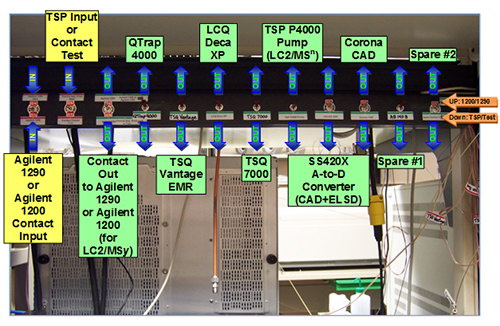
Figure 5. GC×GC chromatogram of the FAME prepared from human colon adenocarcinoma cells HT-29. Reproduced from ref. [13].
GC×GC can be very helpful also in the analysis of many minor compounds (e.g. sterols, stanols, steroids, waxes, etc). Such compounds are usually analyzed after derivatization with N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA)–1% trimethylchlorosilane (TMCS). Slightly polar and moderately polar columns are usually used in the first and second dimension, respectively; since thermally-stable columns in both dimensions are required in relation to the low-volatility of the analytes of interest. These compounds are of interest in many fields of application, therefore, depending on the final purpose, many different sample preparation and derivatization approaches can be exploited. The use of dual MS and FID detection allows one to perform both qualitative and quantitative analysis in a single chromatographic run. Furthermore, the high separation power obtained using GC×GC can play an important role in the simplification of the preparation step. For instance, the entire unsaponifiable fraction of a series of vegetable oils (extra-virgin olive, sunflower, peanut) and dairy products can be subjected to derivatization [BSTFA–1% TMCS], avoiding the tedious thin-layer chromatography step performed for this kind of analysis. Unsaponifiable constituents are nicely spread out on the 2D plane, with each chemical class located in a specific position (Figure 6) [14, 15].
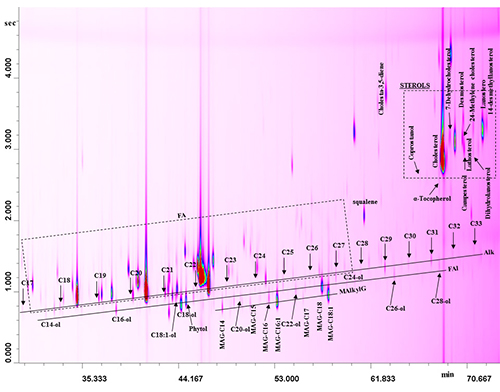
Figure 6. GC×GC chromatogram of the unsaponifiable fraction of a buffalo milk fat. Modified from ref. [15].
An increased number of chemical classes investigated, a better exploitation of the separation space, and the enhancement of the level of information that can be extrapolated from a single chromatographic run can be achieved by choosing the proper preparation method. For instance, if the official method (based on the official International Olive Council method) for fatty acid alkyl esters and waxes is slightly modified by introducing a derivatization step directly on the oil prior to silica column purification, then free sterols, alcohols and other polar minor compounds can be eluted, as TMS-ether moieties, in the same fraction as fatty acid alkyl esters and waxes [16]. The optimization of the chromatographic conditions can be more complicated, since very different chemical classes need to be located in the 2D space, maintaining the chromatographic structure, avoiding wrap-around, and assuring the elution of high boiling compounds in a reasonable time. A compromise was achieved by using a short 1D column, but an oven offset of +25°C was necessary to avoid extensive wrap-around, thus the structure for esterified sterols was lost, since they eluted in the isothermal part of the chromatogram (Figure 7). Using such a method it was possible to comply with the legal requirement of the European legislation regarding the amount of fatty acid alkyl esters and waxes, but at the same time useful diagnostic information regarding the minor components of vegetable oils can be obtained for authentication issues [16].
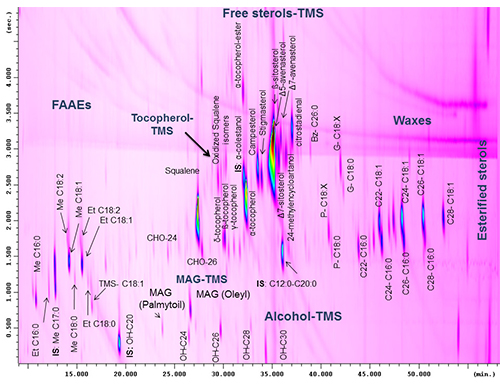
Figure 7. GC×GC chromatogram of minor components of an extra virgin olive oil. Modified from ref. [16].
References
- James, A.T., Martin, A.J.P. Gas-liquid partition chromatography: the separation and micro-estimation of volatile fatty acids from formic acid to dodecanoic acid. Biochem. J., 50, 679-690 (1952).
- Golay, M.J.E. Gas chromatography. New York:Academic press, 1958.
- Liu, Z., Phillips, J.B. Comprehensive two-dimensional gas chromatography using an on-column thermal modulator interface. J. Chromatogr. Sci., 29(6), 227-231 (1991).
- Adahchour, M., Beens, J.; Vreuls, R.J.J., Brinkman, U.A.Th. Recent developments in comprehensive two-dimensional gas chromatography (GC x GC) I. Introduction and instrumental set-up. Trends Anal. Chem., 25(5), 438-454 (2006)
- Murphy, R.E.; Schure, M.R.; Foley, J.P. Effect of sampling rate on resolution in comprehensive two-dimensional liquid chromatography Anal. Chem., 70(8), 1585-1594 (1998).
- Tranchida, P.Q., Purcaro, G., Dugo, P., Mondello, L. Modulators for comprehensive two-dimensional gas chromatography: a review, Trends Anal Chem., 30 (9), 1437-1461 (2011).
- Kinghorn, R.M., Marriott, P.J. Comprehensive two-dimensional gas chromatography using a modulating cryogenic trap J. High Resolut. Chromatogr., 21(11), 620-622 (1998).
- Ledford, E.B.; Billesbach, C.; Termaat, J. Pittcon 2002, March 17-22, New Orleans, LA. contribution #2262P
- Bueno, P.A., Seeley J.V. Flow-switching device for comprehensive two-dimensional gas chromatography. J. Chromatogr. A, 1027, 3-10 (2004).
- Seeley, J.V., Micyus, N.J., McCurry, J.D., Seeley, S.K. Comprehensive two-dimensional gas chromatography with a simple fluidic modulator. Amer. Lab., 38, 24-26 (2006).
- Tranchida P.Q., Franchina F.A., Dugo P., Mondello L., Use of greatly-reduced gas flows in flow-modulated comprehensive two-dimensional gas chromatography-mass spectrometry, J. Chromatogr. A, 1359, 271–276 (2014)
- Mondello, L., Casilli, A., Tranchida, P.Q., Dugo, P., Dugo, G. Detailed analysis and group-type separation of natural fats and oils using comprehensive two-dimensional gas chromatography. J. Chromatogr. A 1019, 187-196 (2003).
- Delmonte P., Fardin-Kia A.R., Rader J.I., Separation of Fatty Acid Methyl Esters by GC-Online Hydrogenation× GC. Anal Chem., 85, 1517-1524 (2013).
- Tranchida, P.Q., Salivo, S., Franchina, F.A., Bonaccorsi, I., Dugo, P., Mondello, L. Qualitative and quantitative analysis of the unsaponifiable fraction of vegetable oils by using comprehensive 2D GC with dual MS/FID detection. Anal. Bioanal. Chem., 405 (13), 4655-4663 (2013).
- Tranchida P.Q., Salivo S., Bonaccorsi I., Rotondo A., Dugo P., Mondello L. Analysis of the unsaponifiable fraction of lipids belonging to various milk-types by using comprehensive two-dimensional gas chromatography with dual mass spectrometry/flame ionization detection and with the support of high resolution time-of-flight mass spectrometry for structural elucidation. J Chromatogr A, 1313, 194–201 (2013).
- Purcaro G., Barp L., Beccaria M., Conte L.S. Fingerprinting of vegetable oil minor components by multidimensional comprehensive gas chromatography with dual detection. Anal. Bioanal. Chem. 407, 309-319 (2015).