Production of Unusual Fatty Acids in Plants
The Author: David Hildebrand, Professor of Plant Biochemistry & Genetics, 403 Plant Science Bldg., 1405 Veterans Dr., University of Kentucky, Lexington, KY 40546-0312, U.S.A.
Introduction
Glycerolipids composed of one, two or three fatty acids esterified to glycerol are a major structural and functional component of cell membranes. Free fatty acids rarely accumulate in healthy living tissues. Cell membranes are mainly composed of diacylglycerols with a polar head group usually a phosphate or galactose derivative. Most storage lipids are composed of triacylglycerols. Some storage tissues such as seeds can be up to 75% triacylglycerols and some fruits can have triacylglycerols at almost 90% of dry weight (e.g. oil palm)! Most leaves highly active in photosynthesis contain about 6% lipid of which ~57% is glycerolipid and ~25% chlorophyll.
The main storage lipid is triacylglycerol and the main polar lipids are phospholipids and glycolipids. The distribution of these different lipids in Arabidopsis leaves, roots and seeds is shown in Table 1. Oilseeds are often ≥ 95% triacylglycerols with cytosolic phospholipids making up most of the remainder. Leaves and roots normally have little or no detectable triacylglycerols. Cytosolic phospholipids dominate lipids of nonphotosynthetic tissues such as roots whereas galactolipids (galactose-containing glycolipids), are most abundant in photosynthetically active leaves (Table 1).
| Table 1. Lipid distributions of Arabidopsis seed, leaf and root tissues | |||
| Lipid Type | Seed | Leaf | Root |
| Plastid glycolipids | 1% | 60% | 5% |
| Plastid phospholipids | 1% | 16% | 12% |
| Cytosolic phospholipids | 3% | 24% | 83% |
| Triacylglycerols | 95% | 0% | 0% |
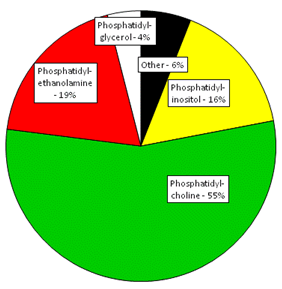
Plastid glycolipids and phospholipids, mainly in thylakoids, make up 76% of leaf lipids. The main phospholipids in plants are phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, phosphatidylinositol, phosphatidylserine and cardiolipin. The relative abundance of different phospholipids in developing soybeans is typical of nonphotosynthetic plant tissues. These are dominated by phosphatidylcholine at 50-60% followed by phosphatidylethanolamine and phosphatidylinositol (Fig. 1).
Figure 1. Relative amounts of individual phospholipids in developing soybean seeds. Adapted from (Slack et al., 1978).
The principal glycolipids in order of abundance in chloroplasts are monogalactosyldiacylglycerol, digalactosyldiacylglycerol and sulfoquinovosyldiacylglycerol (Table 2). Five fatty acids (18:1, 18:2, 18:3, 16:0 and in some species 16:3) make up over 90% of acyl chains of structural glycerolipids of most plant membranes and seed oils. The major fatty acids of plant tissues (and most other eukaryotic organisms) have a chain length of 16 or 18 carbons and contain from zero to three cis-double bonds. α-Linolenic acid (18:3(n-3)) is the most abundant fatty acid in all chloroplast lipids especially mono- and digalactosyldiacylglycerols. Digalactosyldiacylglycerols and especially monogalactosyldiacylglycerols are also relatively high in 16:3 in so-called ‘16:3 plants’ such as Arabidopsis. Monogalactosyldiacylglyerols' fatty acids are nearly all polyunsaturated and digalactosyldiacylglycerol only has small amounts of 16:0. Sulfoquinovosyldiacylglycerol has a relatively high amount of the saturated fatty acid 16:0. Phosphatidylglycerol is unusual in that it has a high level of trans-∆3-16:1 (Table 2). Further data are available in a separate web page on the composition of plant lipids.
| Table 2. Fatty acid composition of total Arabidopsis leaf lipids and major lipid fractions of spinach chloroplasts | |||||||
| Plant tissue/lipid | Fatty acid | ||||||
| 16:0 | 16:1t | 16:3 | 18:0 | 18:1 | 18:2 | 18:3 | |
| Arabidopsis leaves | 15.4 | 3.4 | 16.2 | 1.1 | 2.6 | 14.1 | 47.3 |
| MGD | <1 | <1 | 22 | <1 | 1 | 5 | 75 |
| DGD | 3 | <1 | 10 | <1 | 2 | 3 | 82 |
| SQD | 44 | <1 | <1 | <1 | <1 | 5 | 51 |
| PG | 12 | 24 | 2 | <1 | 2 | 6 | 55 |
| MGD = monogalactosyldiacylglycerol; DGD = digalactosyldiacylglycerol; SQD = sulfoquinovosyldiacylglycerol; PG = phosphatidylglycerol. | |||||||
Higher plants can be classified as 16:3 or 18:3 plants. Some 16:3 plants contain significant amounts (5-10%) of cis-7,10,13-hexadecatrienoic acid (16:3(n-3)) in leaf lipids mainly in monogalactosyldiacylglycerol (where it is <20% of the fatty acids) and also in digalactosyldiacylglycerol (Table 2). 18:3 plants do not contain detectable levels of 16:3. 16:3 is not known to accumulate in the seed oil even in 16:3 plants such as Arabidopsis or Brassica spp. (Table 3). In analyses of the fatty acid compositions of the oils of 468 plant species in 141 families, it was found that only 12% of angiosperm species are 16:3 plants. 16:3 is ubiquitous among lower eukaryotic land plant species examined of the Bryophytes, Pteridophytes, Gymnosperms, Prespermatophytes, Chlamydosperms, Gnetaceae and Ephedraceae. Common high 16:3 crop plant families include the Brassicaceae and Solanaceae, while species of the Chenopodiaceae, such as spinach, include 16:3 plants. The most important crop plant families, Poaceae (Graminaceae) and Fabaceae (Leguminaceae), are 18:3 plants, but all nine species of the very important fruit family, Rosaceae, examined have small amounts of 16:3.
|
Table 3. Fatty acid composition among some important plant oils |
||||||
| Plant | Fatty Acid | |||||
| 16:0 | 18:0 | 18:1 | 18:2 | α-18:3 | Other | |
| Arabidopsis thaliana seeds | 9 | 4 | 19 | 27 | 20 | 21 |
| Coconut (Cocos nucifera) | 9 | 3 | 6 | 2 | <1 | 80 |
| Cocoa butter (Theobroma cacao) | 27 | 35 | 35 | 3 | <1 | <1 |
| Palm (Elaeis guineensis) | 46 | 4 | 38 | 10 | <1 | 2 |
| Olive (Olea europaea) | 11 | 2 | 76 | 8 | 1 | 2 |
| Canola (Brassica napus) | 4 | 2 | 59 | 21 | 10 | 3 |
| Peanut (Arachis hypogea) | 10 | 2 | 48 | 34 | <1 | 6 |
| Sesame (Sesamum spp.) | 7 | 4 | 41 | 43 | <1 | 3 |
| Corn (Zea mays) | 11 | 2 | 25 | 61 | 1 | <1 |
| Soybean (Glycine max) | 11 | 4 | 24 | 54 | 7 | 1 |
| Sunflower (Helianthus annuus) | 7 | 3 | 21 | 69 | <1 | <1 |
| Cottonseed (Gossypium spp.) | 24 | 2 | 18 | 54 | <1 | 2 |
| Safflower (Carthamus tinctorius) | 7 | 2 | 12 | 78 | <1 | 1 |
| Flax (Linum usitatissimum) | 5 | 5 | 26 | 18 | 46 | <1 |
| Chia (Salvia hispanica) | 7 | 3 | 7 | 21 | 61 | <1 |
Most plant seeds accumulate storage products, such as starch or triacylglycerols, during seed development to provide nutrients and energy for seedling establishment. Many seed crops such as corn, wheat, rice, peas and common beans (Phaseolus vulgaris) accumulate starch as the main form of energy storage in the seeds, although the embryo or germ portion is often high in oil. Oilseeds such as soybean (Glycine max), canola (Brassica napus), sunflower (Helianthus annuus), cotton seed (Gossypium spp.), peanuts (Arachis hypogea) and many other seeds accumulate oil instead of starch. Like many minute seeds, Arabidopsis thaliana and tobacco (Nicotiana tabacum) seeds accumulate oil as an energy store with Arabidopsis seeds usually being ~42% oil. The fatty acid composition of major oilseeds in comparison to the model plant, Arabidopsis thaliana, indicates that the seed oil triacylglycerol is dominated by the five main membrane fatty acids with some exceptions (Table 3). However the relative distribution of fatty acids in seed oils varies widely among plant species.
Major oilseeds can be divided into four groups based on fatty acid composition with some dominated by saturated fatty acids such as coconut and palm, some by monounsaturated fatty acids such as olive, some by linoleic acid such as soybean and sunflower oils and some by the ω3 fatty acid, α-linolenic acid such as flax or linseed oil. High oleate options of the normally high linoleate oils including sunflower and soybean oils are now available. This makes such oils more stable oxidatively especially as cooking oils. The main exceptions are coconut and palm kernel triacylglycerols, which are dominated by medium-chain fatty acids of 8 to 14 carbons in length. The main “other” fatty acids normally present in Arabidopsis seed oil are longer-chain fatty acids particularly eicosenoic acid, 22:1. Many other members of the Brassicaceae family in addition to Arabidopsis accumulate longer-chain fatty acids in the seed oil. Normal rapeseed (Brassica napus) seed oil for example is as much as 50% of the 22-carbon monounsaturated fatty acid, erucic (docosenoic) acid. Canola is a low erucic acid rapeseed mutant, which also exhibits reduced glucosinolate content. Erucic acid has industrial value but may cause health problems if consumed in large quantities.
There is a wide and diverse range of unusual fatty acids in the seed oil of many wild plants. Plant species that accumulate such acids are mostly unsuitable for modern agricultural practices. Surveys of the fatty acid composition of seed oils from different species have identified more than 200 naturally occurring fatty acids of which 18 representatives are listed (Table 4).
|
Table 4. Some unusual fatty acids (UFA) that accumulate in seed oils and examples of seeds with high levels |
|||
| Common name | Chemical name | High accumulator | % UFA |
| alchornoic | 14-epoxy,cis-11-eicosenoic | Alchornea cordifolia | 50 |
| axillarenic | 11,13-dihydroxy-tetracos-trans-9-enoic | Baliospermum axillare | 3 |
| calendic | trans-8,trans-10,cis-12-octadecatrienoic | Calendula officinalis | 63 |
| catalpic | trans-9,trans-11,cis-13-octadecatrienoic | Catalpa bignonioides | |
| dimorphecolic | 9-hydroxy-trans,10-trans-12-octadecadienoic | Dimorphotheca pluvialis | 60 |
| coronaric | 9-epoxy,cis-12-octadecenoic | Chrysanthemum coronarium | |
| crepenynic | octadec-cis-9-en-12-ynoic | Crepis alpina | 74 |
| eleostearic | cis-9,trans-11,trans-13-octadecatrienoic | Aleurites fordii | 80 |
| epoxystearic | 9-epoxy-octadecanoic | Tragopogon porrifolius | 3 |
| isanolic | 8-hydroxy-octadec-17-en-9,11-diynoic | Ongokea gore | |
| isoricinoleic | 9-hydroxy-12-cis-octadecenoic | Wrightia coccinea | 76 |
| licanic | 4-oxo-cis-9,trans-11,trans-13-octadecatrienoic | Licania rigida | 78 |
| lesquerolic | 14-hydroxy-cis-11-eicosenoic | Lesquerella fendleri | 55 |
| parinaric | cis-9,trans-11,trans-13,cis-15-octadecatetraenoic | Parinarium laurunum | 54 |
| punicic | cis-9,trans-11,cis-13-octadecatrienoic | Punicia granatum | 86 |
| phloionolic | 9,10,18-trihydroxyoctadecanoic | Chamaepeuce afra | 9 |
| ricinoleic | 12-hydroxy-9-cis-octadecenoic | Ricinus communis | 88 |
| vernolic | 12-epoxy,cis-9-octadecenoic | Vernonia galamensis | 80 |
The number and arrangement of double or triple bonds and various functional groups, such as hydroxyls, ketones, epoxyls, cyclopentenyl or cyclopropyl groups, furans, or halogens, can vary. Some unusual fatty acids might disrupt membrane structure and function if incorporated into membrane-forming lipids. Results of studies with Escherichia coli show that branched-chain, brominated, and trans-unsaturated fatty acids support growth, some of which are in fact "usual" fatty acids for this organism. These studies show that at least some unusual fatty acids can exist in some functional membranes, but it remains quite possible that their inclusion in normal plant membranes would be deleterious. The biological role of most of these "unusual" fatty acids is not known, but it is thought that many may have a role in defence against pests as do many "secondary products" in plants. Examples of the best-characterized unusual fatty acids in plants will be briefly reviewed here with more detailed information on hydroxy oils in plants covered in the AOCS Lipid Library document by Mark Smith and Tom McKeon.
Medium-Chain Fatty Acids
Medium-chain fatty acids such as lauric acid and derivatives are highly valued for their detergent properties and are used extensively in soaps, shampoos and detergents. Traditionally, soaps were made from animal fats by converting the fatty acids of their triacylglycerols, mainly stearate or palmitate, to sodium and potassium salts. Salts of these longer-chain fatty acids are not very soluble in water especially at colder temperatures. Lower temperatures reduce their detergent properties and salts of short-chain fatty acids are not hydrophobic enough for efficient cleansing of hydrophobic materials. Salts of medium-chain fatty acids such as dodecanoic acid or lauric acid are ideally balanced in hydrophobicity and water solubility for use in cleaning materials. A very effective and commonly used detergent is a derivative of lauric acid, sodium dodecylsulfate (SDS), for example:
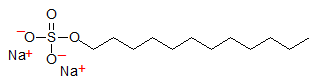
Most lauric acid is derived from plants of the palm family (Arecaceae), especially coconut and oil palm, and major temperate oilseeds contain little or no laurate. Because lauric acid is mainly derived from only two crop species and mainly from one region in the world, climatic conditions and other factors can greatly affect the supply and therefore the price. Hence there has long been an interest in temperate crop sources that can be grown in places such as the United States, Canada and Europe. A number of plants have been identified in nature that accumulate laurate and/or other medium-chain fatty acids in their seed oils including Attalea colenda, another palm, and Cuphea species of the Lythraceae family. Work is in progress on the domestication of Cuphea as a temperate source of medium-chain fatty acids. Fruits of Laurus nobilis of the Mediterranean region are ~45% lauric acid. The seeds of the temperate tree, the California laurel (Umbellularia californica Nutt., Lauraceae), are reported to have as much as 64% oil containing up to 70% lauric acid with much of the remaining oil fatty acids being capric acid. Qualea grandiflora (Vochysiaceae) from a Brazilian savannah or 'cerrado' can contain over 70% lauric acid. Mature seeds of another Lauraceae species, Actinodaphne hookeri, contain as much as 95% laurate in their oil.
Specific thioesterases catalyse the removal of newly formed medium-chain acyl groups from acyl-acyl carrier protein (ACP) and prevent them from being used by the prokaryotic pathway of glycerolipid synthesis because the sn-glycerol-3-phosphate acylating enzymes require an acyl-ACP substrate. In the endoplasmic reticulum, the partitioning of the medium-chain acyl groups between membrane and storage lipid might be achieved by acyltransferases. In Cuphea, sn-glycerol-3-phosphate acyltransferase can use both medium-chain and common fatty acids. However, lysophosphatidic acid acyltransferase (LPAT; also known as LPAAT) from Cuphea preferentially uses medium-chain fatty acids. In the final step of triacylglycerol synthesis, diacylglycerol acyltransferase (DGAT) preferentially uses 10:0/10:0-diacylglycerol species and almost exclusively takes a medium-chain acyl-CoA. Thus, medium-chain fatty acids are efficiently incorporated into triacylglycerol in the developing seeds of Cuphea. In contrast, cholinephosphate acyltransferase (CPT) was not found to be involved the selective accumulation of medium-chain fatty acid in triacylglycerol. However, selective removal of laurate from the sn-2 position of phosphatidylcholine was found, which might have been due to a specialized phospholipase A2 or a specialized phospholipid:diacylglycerol acyltransferase (PDAT).
Studies of novel fatty acid production by transgenic plants have provided new insight on the segregation of the novel fatty acid from membrane lipids. For example, rapeseeds that express a California bay tree (Umbellularia californica) medium-chain fatty acid thioesterase (FatB1) using the seed-specific Napin promoter can produce high amounts of laurate. In some of these transgenic lines, although only 6 mol% laurate was found in phosphatidylcholine of the membrane lipids of mature seeds that had 55 mol% of laurate in the seed oil, the laurate levels in phosphatidylcholine of the membrane lipids of developing seeds could be as high as 46 mol% instead of the 1 to 4 mol% found in natural laurate producers. This study demonstrates the relative ineffectiveness of novel fatty acid segregation from membrane lipids in transgenic producers compared to natural producers. The main limitation to higher levels is the near exclusion of laurate from the sn-2 position by specificity of the lysophosphatidic acid acyltransferase.
Coconut endosperm triacylglycerol is typically ~50% laurate distributed in all three positions indicating that coconut endosperm LPAT can accept laurate-CoA as a substrate. The Calgene group therefore purified the coconut endosperm LPAT and cloned its corresponding cDNA. Expression of this LPAT together with the California bay thioesterase in canola resulted in oil with significant amounts of laurate in the sn-2 position, but the overall laurate content of the oil was not increased. In addition, transgenic oilseed rape that accumulates 60 mol% lauric acid has high activities of β-oxidation and glyoxylate enzymes apparently triggered by the accumulation of laurate in membrane lipids and this may in turn up-regulate fatty acid biosynthesis forming a futile cycle.
When the FatB1 thioesterase is expressed in oilseed rape under the control of the 35S promoter, medium-chain fatty acids can only be found in seeds even though higher FatB1 activity levels are found by in vitro analysis of isolated chloroplasts of vegetative tissues. The medium-chain fatty acids are probably produced in the leaves and then rapidly broken down because medium-chain fatty acid-targeted enzymes of both the β-oxidation pathway and the glyoxylate cycle pathways are induced in the leaves of the transgenic plants. Secretion of medium-chain fatty acids by E. coli mutants blocked in β-oxidation expressing FatB1 further supports the above hypothesis. Interestingly the overall oil content was not significantly reduced apparently due to higher overall triacylglycerol biosynthesis induced in these transgenic plants to make up for the laurate lost to β-oxidation. How to achieve very high accumulation (i.e. close to 100%) of unusual fatty acids in oilseeds remains a major challenge of plant biochemistry and metabolic engineering. Nevertheless, the high-laurate canola is a remarkable achievement and has as high or higher laurate than normal sources of plant oils for production of detergents.
Very-Long-Chain Fatty Acids
Very-long-chain fatty acids (VLCFAs) are also excluded from the membrane lipids in their natural producers. For example, only 3 mol% of erucic acid is found in the phospholipids of Crambe abyssinica that contains more than 60% of erucic acid in mature seed oil. Radiochemical feeding studies indicate that VLCFA-CoA is excluded but oleoyl-CoA was accepted for phospholipid synthesis. However, unlike medium-chain fatty acids-producing rapeseed, transgenic Arabidopsis expressing a fatty acid elongase 1 (FAE1) under the control of the 35S promoter accumulated up to 30 mol% of VLCFAs in the phospholipids of the vegetative tissues, which caused changes in plant morphology and organellar structures with the severity of these changes strongly correlated with the levels of VLCFA accumulation.
In contrast, the VLCFAs were excluded from membrane lipids in the developing seeds of transgenic Arabidopsis. The inefficient catabolism of VLCFAs in nonseed tissues in this instance may have resulted in their accumulation in these tissues. It is speculated that VLCFAs may play a role in membrane bilayer structure/function so that they are not catabolized, by analogy with the presence of C24 to C26 VLCFAs in sphingolipids. One possibility is that VLCFAs may be required for the formation of highly curved membrane structures, which may mimic the structural role of sphingolipids. The yeast Slc mutant, which lacks sphingolipids synthesis, can survive without sphingolipids by making novel glycerolipids containing C26 fatty acids, strongly supporting this hypothesis. This yeast gene supports VLCFA accumulation in plants also. Expression of a nasturtium (Tropaeolum majus) acyl-CoA-dependent diacylglycerol acyltransferase 1 (DGAT1) gene in high-erucic acid rapeseed or canola did not increase the erucic acid levels but significantly increased oil content. Another DGAT or genes encoding additional enzymes may be needed for very high erucic acid accumulation in commercial oilseeds (see below).
Novel Monoenoic Fatty Acids
Vegetable oils rich in monounsaturated fatty acids (MUFA) not only are important in human nutrition but also can be used as renewable sources of industrial chemicals. Palmitoleic acid, cis-9-hexadecenoic acid, (16:1Δ9), has the nutritional and industrial chemical advantages of the much more common longer-chain MUFA, oleic acid (18:1Δ9), but with much better cold flow properties. 16:1Δ9 is considered healthier than the common monounsaturated fatty acid, oleic acid. Sea buckthorn (Hippophae rhamnoides) and cat's claw, Macfadyena unguiscati (formerly, Doxantha unguiscati L.) have high levels of 16:1Δ9, accumulating to 40% and 80% of the mature seed oil. Macadamia (Macadamia integrifolia) nut oil is the main commercially available source of palmitoleic acid. Its oil is unique among edible sources in that monounsaturated fatty acids are the predominant component (about 80%) and a considerable portion (17-21%) of this is palmitoleic acid (a component not present in substantial amounts in olive oil).
Novel monoenoic fatty acids, such as petroselinic acid (18:1Δ6) are synthesized while esterified to ACP and selectively hydrolyzed by specific ACP thioesterases. Free monoenoic fatty acids move out of plastids to the endoplasmic reticulum for triacylglycerol synthesis. In developing coriander and Thunbergia seeds, petroselinic acid was found to cycle through phosphatidylcholine, most likely at the sn-2 position before entering triacylglycerol. Transgenic rapeseed producing petroselinic acid can induce selective catabolism of petroselinic acid by β-oxidation and the glyoxylate cycle.
γ-Linolenic and Stearidonic Acids
γ-Linolenic acid (Δ6,9,12-18:3) and stearidonic acids (octadecatetraenoic acid, Δ6,9,12,15-18:4) are synthesized from linoleic acid (Δ9,12-18:2) and α-linolenic acids (Δ9,12,15-18:3), respectively. Both fatty acids are of importance as pharmaceuticals and general health supplements, and occur in several plant families including members of the Boraginacae and Onagraceae. Microsomes prepared from developing borage cotyledons actively catalyze the desaturation of [14C]-linoleate to γ-linolenate and [14C]-γ-linolenate to octadecatetraenoate. This activity is dependent upon NADH, and the substrate appears to be esterified to the sn-2 position of phosphatidylcholine. A cDNA from borage that encodes a Δ6-fatty acid-desaturase was identified. The amino acid sequence of this desaturase is distinct from that of other higher plant desaturases in that it has an N-terminal extension that contains a cytochrome b5-like domain, complete with a diagnostic heme-binding motif.
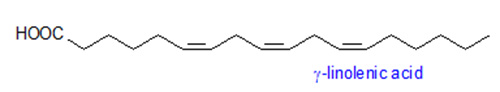
Introduction of the borage Δ6-desaturase in tobacco and Arabidopsis resulted in high levels (>20%) of γ-linolenic acid in early stages of seed development but these levels declined significantly to <2% by seed maturity. Up to 29% stearidonic acid and >10% γ-linolenic acid were achieved in soybean seeds by co-expression of a borage Δ6-desaturase and Arabidopsis ω3-desaturase. Flax seeds with >13% stearidonic acid with no accumulation of γ-linolenic acid can be obtained by expression of a gene encoding a Δ6-desaturase from Primula vialii. This lack of γ-linolenic acid may be desirable for some ω3 health uses of stearidonic acid sources.
Δ12 Desaturase-like Enzymes and Their Use in the Modification of Fatty Acid Residues
Structures and Functions
Fatty acid desaturases in plants catalyze the introduction of cis-double bonds into acyl chains with regiospecificity. Adjacent double bonds are methylene-interrupted. However, some plants have evolved new functions for their desaturases. These new enzymes are termed 'desaturase-like' due to their similarities to desaturases. Desaturase-like enzymes create new functional groups for fatty acids such as conjugated double bonds, hydroxy, epoxy, and acetylenic groups although a cytochrome P450 epoxygenase from Euphorbia is responsible for the epoxidation of sn-2 linoleoyl-phosphatidylcholine. Other than acting typically on Δ12 positions of the acyl groups, some desaturases can also act on an existing Δ9 double bond. Although only one reaction product results from most of these enzymes, a desaturase/hydroxylase bifunctional enzyme from Lesquerella has been identified. The resulting "novel" fatty acids are just a small portion out of hundreds of diverse fatty acid species known to be produced in the plant kingdom. Most of the desaturase-like enzymes identified to date are encoded by diverged Fad2 genes. Such genes include hydroxylases from Ricinus communis and Lesquerella fendleri, epoxygenases from Vernonia galamensis and Crepis palaestina, acetylenase from Crepis alpina, and conjugases from Mormordica charantia, Impatiens balsamica, and Calendula officinalis.
Similar to desaturation, the reactions for hydroxylation, epoxidation, and acetylenation of fatty acids involve first the enzymatic removal of a hydrogen atom from a methylene group in an acyl chain. Because breaking of the C-H bond of a methylene group requires ~98 kcal/mol, an activated oxygen and a metal cofactor are needed for initiation of the reactions. A short electron transport chain is also required for these reactions. In the endoplasmic reticulum, the electron transport consists of cytochrome b5 and cytochrome b5 reductase and the electron donor is NADH. Most desaturase-like enzymes identified thus far are endoplasmic reticulum-associated non-heme diiron proteins with a consensus motif that is composed of histidines (HX3-4H, HX2-3HH, (H/Q)X2HH) that are in equivalent hydrophilic domains separated by equivalent hydrophobic domains and positioned on the cytoplasmic face of the membrane.
Analysis of the phylogenetic relationships of acetylenase, conjugase, epoxygenase, hydroxylase and desaturase amino acid sequences indicates that acetylenases, conjugases, epoxygenases and hydroxylases group semi-randomly among Δ12 desaturase sequences indicating that these enzymes arose independently many times from pre-existing desaturases during plant evolution. Some ω3 desaturases also are highly homologous to Δ12 desaturases indicating that ω3 desaturases evolved from Δ12 desaturases also.
Conjugated Fatty Acids
Among the conjugated fatty acids that occur in plants, the best known is probably eleostearic acid from the tung plant, Aleurites fordii (Euphorbiaceae), where it comprises 80% of total fatty acids. α-Eleostearic acid is 9-cis,11-trans,13-trans-octadecatrienoic acid and is responsible for the industrial importance of tung oil as an excellent drying oil. The tung conjugase that makes eleostearic acid from linoleic acid is a desaturase analogue and has been cloned and expressed in yeast. Other plants in the genus Aleurites are also significant sources of eleostearic acid such as Aleurites montana (67%) and Aluerites trisperma (38%).
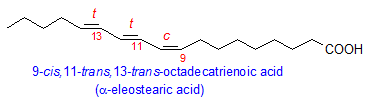
There are many other sources of eleostearic acid. One of the most important is the Chinese bitter gourd, Momordica charantia, a crop plant throughout Asia. Seeds of his plant contain up to 68% eleostearic acid. Other plant sources include Ricinodendron heudelotti (up to 56%), Fevillea cordifolia (31%), and Parinari montana (36%).
Calendic acid (trans-8,trans-10,cis-12-octadecatrienoic acid) is found throughout the genus Calendula of the Asteraceae. Calendula officinalis has been investigated as a new oil crop and it has a fatty acid profile of 62.8% calendic acid. The enzyme responsible for the formation of calendic acid from linoleic acid has been isolated and cloned into yeast and somatic soybean embyros. This enzyme (called a conjugase) acts on the double bond at position 9 in linoleic acid to make the conjugated trans-Δ8,Δ10 double bonds.
Punicic acid (cis-9,trans-11,cis-13-octadecatrienoic acid) was first isolated from pomegranate seeds (Punicia granatum) at 86%. It also occurs in several species of Trichosanthes, up to 52% in Trichosanthes nervifolia. The fatty acid in the Trichosanthes was not recognized as punicic acid in early reports and was called trichosanic acid. Other plants that have punicic acid include Mormodica balsamina at 50%, Diplocyclos palmatus (38.2%) and Apodanthera undulata (30% by weight).
Genes encoding conjugases have been cloned from both pomegranate and Trichosanthes kirilowii and expressed in yeast and Arabidopsis. The enzyme was Δ12 desaturase-like and acted on the 12 position double bond in linoleic acid, creating the trans-11,cis-13 bonds of the conjugated triene. Interestingly, the conjugase was also found to be bifunctional and exhibited Δ12 desaturase activity.
Parinaric acid (cis-9,trans-11,trans-13,cis-15-octadecatetraenoic acid) was first isolated from the seed of Parinarium laurinum at a level of 53.5%. It is also found in several species of Impatiens, including Impatiens edgeworthii (48%), I. balsamina (29.1%), I. capensis (42%) and I. pallida (30%), and also in Sebastiana brasiliensis (39%). Parinaric acid is used as a fluorescent probe in membrane research.
Licanic acid (4-oxo-cis-9,trans-11,trans-13-octadecatrienoic acid) is a keto-isomer of eleostearic acid and was first isolated from oiticica oil, from the plant Licania rigida at 78.2%. Couepic acid from the seed of Couepia grandiflora was found to be identical to licanic acid.
Catalpic acid (trans-9,trans-11,cis-13-octadecatrienoic acid) is an isomer of eleostearic acid isolated first from Catalpa bignonioides. A conjugase has been cloned from Catalpa ovata.
Epoxy and Acetylenic Fatty Acids
Epoxy fatty acids can have many industrial applications. Certain genotypes of several plant species accumulate high levels of such fatty acids in the seed oil. Epoxy fatty acids, such as vernolic (cis-12,13-epoxyoctadeca-cis-9-enoic) and coronaric (cis-9,10-epoxyoctadeca-cis-12-enoic) acids, have been found as components of the seed oil of species represented by a number of plant families, including the Asteraceae, Euphorbiaceae, Onagraceae, Dipsacaceae, and Valerianaceae. Vernolic acid was first elucidated in Vernonia anthelmintica seed at 65-75%, and subsequently in V. galamensis at ~80%, and V. volkameriaefolia at 63.5%. Vernolic acid has also been found in Stokesia laevis (70%), several Crepis spp. (18%-68%), Erlangea tomentosa (52%), Centratherum ritchiei (30%), Euphorbia lagascae (57%) and Bernardia pulchella (91%). Of these, E. lagascae and V. galamensis have been investigated for possible crop use. Many other plants also have lower but detectable vernolic acid levels.
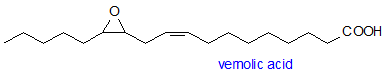
Coronaric acid was first isolated from Chrysanthemum coronarium. Many other plants since then have been reported to contain this acid, including Xeranthemum annuum (8%), Lactuca sativa (16.9%) and several Acacia species (3.5%-6.8%). Interestingly, sunflower seed showed the presence of coronaric acid (2%) after prolonged storage. With Cichorium intybus after prolonged storage, the total oxygenated fatty acids increased from 1% to 17% and were found to consist of conjugated hydroxy fatty acids and coronaric acid.
Little work has been done with the other epoxy fatty acids found in nature. These acids include 9,10-epoxystearic acid from Tragopogon porrifolius (3%) and alchornoic acid (14,15-epoxy-cis-11-eicosenoic acid) from Alchornea cordifolia (50%). Other epoxy fatty acids, such as 15-epoxy-cis-9,cis-12-octadecadienoic acid occur in small amounts in a few species. These epoxy fatty acids (like coronaric and epoxystearic acid) may be formed during seed storage.
The Stymne group provided good evidence that a P-450 monooxygenase enzyme catalyzes vernolic acid biosynthesis in Euphorbia lagascae. The Euphorbia enzyme utilized linoleoyl-phosphatidylcholine and other phospholipids as the substrate producing vernoleoyl-phosphatidylcholine. The vernoleate did not accumulate in the phosphatidylcholine, only in triacylglycerols. However, assays with Vernonia extracts indicated some fundamental differences: (1) CO apparently inhibited the activity but less so than the Euphorbia enzyme; (2) both NADH and NADPH were necessary for activity and both supported the activity to about the same extent; (3) the activity was inhibited by cyanide; (4) the activity was NOT inhibited by anti-cytochrome P-450 reductase antibodies; (5) the activity was inhibited by anti-cytochrome b5 antibodies. These results confirm that the Vernonia epoxygenase is distinctly different from both usual P-450 monooxygenases and from Euphorbia epoxygenase. This suggests that the ability to synthesize vernolic acid has arisen independently throughout evolution.
A gene encoding Δ12 epoxygenase was first isolated from a species of Crepis, C. palaestina, and was found to be a Δ12 desaturase-like enzyme. This gene was expressed in Arabidopsis and yeast. Epoxygenase genes from S. laevis and V. galamensis have also been cloned. The epoxygenase gene from Euphorbia lagascae was isolated and found to be a cytochrome P450 consistent with the prior biochemical studies mentioned above.
Acetylenic bonds are present in more than 600 naturally occurring compounds. The seed oil of Crepis alpina contains about 70% of the acetylenic fatty acid, crepenynic acid (octadec-9-en-12-ynoic acid). It might be speculated that the acetylenic fatty acids could be derived from epoxy fatty acid, followed by two dehydration steps, yielding first the enol and then the acetylenic fatty acid.
The Δ12 epoxygenation and Δ12 acetylenation enzymes of Crepis have biochemical characteristics typical of fatty acid desaturases. A gene encoding an epoxygenase gene of C. palaestina and a gene encoding an acetylenase gene of C. alpina were isolated using a Δ12 desaturase-like sequence as a probe. Both enzymes have characteristics similar to the membrane proteins containing non-heme iron that have histidine-rich motifs.
Genetic Engineering of Oilseeds for Unusual Fatty Acid Accumulation
As mentioned above, in most cases, sources of these unusual fatty acids cannot be produced economically on a commercial scale. Hydroxy, epoxy, conjugated, acetylenic, very-long-chain, medium-chain and branched-chain fatty acids and liquid waxes are among the industrial targets of greatest interest. Oils high in hydroxy fatty acids can be produced from castor and Lesquerella, but it could be produced more economically on a large scale currently with canola or soybeans engineered with genes for such metabolism due to the value of the meal co-product and better-developed agronomic properties. Genes for most of these unusual fatty acids have been cloned and good reviews have been written on this subject. It is easy to produce unusual fatty acids in transgenic oilseeds with the genes encoding enzymes for unusual fatty acid biosynthesis, but it has been very difficult to achieve accumulation of unusual fatty acids to more than 10% of the total lipids. This is in contrast to the accumulation of as much of 95% of the seed oil triacylglycerols being composed of a single unusual fatty acid such as the hydroxy fatty acid ricinoleate in castor oil, the epoxy fatty acid vernoleate in Bernardia pulchella oil and the short-chain fatty acid caproate in Cuphea koehneana oil.
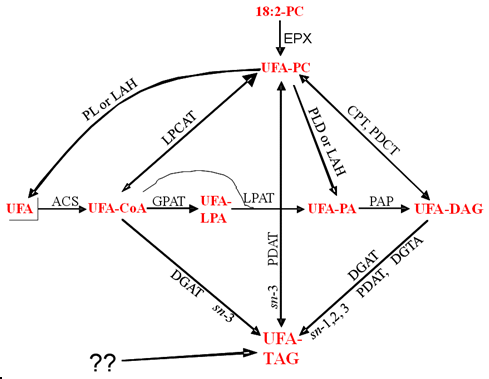
In the cases where the details of the biosynthesis of the unusual fatty acids are known, they are made from phosphatidylcholine in the endoplasmic reticulum but then selectively accumulate in seed oil triacylglycerols. They do not accumulate in membrane lipids such as the starting phosphatidylcholine in the plants that accumulate high levels in triacylglycerols, but do not show such selective distribution in transgenic oilseeds with unusual fatty acid biosynthetic genes alone. This has led to studies on whether triacylglycerol biosynthetic enzymes might have selectivity for such fatty acids that accumulate in the triacylglycerols. Possible routes for this selective accumulation in triacylglycerols are illustrated in Figure 2.
Figure 2. Possible routes for the selective accumulation of unusual fatty acids in seed oils.
Schematic pathways for unusual fatty acid (UFA) incorporation into triacylglycerols (TAG) after synthesis in phosphatidylcholine (PC). The sn numbers on a pathway indicate the possible positions of vernoloyl groups in TAG. LPA, lysophosphatidate; PA, phosphatidate; PDAT, phospholipid:diacylglycerol acyltransferase; CPT, cholinephosphotransferase; DGAT, acyl-CoA:diacylglycerol acyltransferase; DGTA, diacylglycerol transacylase; LPCAT, lysophosphatidylcholine acyltransferase; GPAT, glycerol-3-phosphate acyltransferase; LPAT, lysophosphatidate acyltransferase; PAP, phosphatidate phosphatase; PL, phospholipase; ACS, acyl-CoA synthetase.
In order to induce large changes in oil composition, lysophosphatidic acid acyltransferase (LPAT) has been considered an important target enzyme because of its selective discrimination ability. High-erucic acid rapeseed (HEAR; Brassica napus) and meadowfoam (Limnanthes) have 60% and 90% erucic acid in their triacylglycerols. In meadowfoam, erucic acid is present in the sn-2 position of triacylglycerols whereas it is excluded in rapeseed. This difference was attributed to the substrate specificity of LPAT in the two species. To alter the stereochemical composition of rapeseed oil, a cDNA encoding Limnanthes seed-specific LPAT was expressed in Brassica napus plants using a napin expression cassette. In the transgenic plants, 22.3% erucic acid was present at the sn-2 position leading to the production of trierucin. However, alteration of erucic acid at the sn-2 position did not affect the total erucic acid content. It may be that the meadowfoam LPAT does not increase the erucic acid content of rapeseed because of the limited pool size of the 22:1 coenzyme A in the maturing embryos of B. napus.
The metabolism of laurate was found to be different in transgenic B. napus lines (transformed with a California bay lauroyl-acyl carrier protein thioesterase cDNA driven by napin promoter) and the natural laurate accumulators coconut, oil palm and Cuphea wrightii. When tested at the midstage of embryo development, the phosphatidylcholine had up to 26 mole% of laurate in the transgenic rapeseed high laurate line, whereas in other species it ranged between 1 and 4 mole%. The laurate in the Brassica triacylglycerols was almost totally confined to the outer sn-1 and sn-3 positions whereas the laurate in coconut and Cuphea was highest in the sn-2 position. Very low amounts of laurate were found in the sn-2 position in diacylglycerols and phosphatidylcholine of the rapeseed lipids indicating no arrangement of laurate between the outer and sn-2 positions occurred in any of the lipids. There was an enhanced activity of lauroyl-phosphatidylcholine metabolizing enzymes in the laurate-producing rapeseed when embryos were fed with 14C-lauroyl-phosphatidylcholine and 14C-palmitoyl-phosphatidylcholine. The data indicated that diacylglycerols were preferentially utilized from natural laurate accumulators like oil palm, coconut and Cuphea. Transgenic rapeseed oil expressing California bay thioesterase produced 60% saturated fatty acids, with laurate alone comprising 48%. In these plants laurate was present only at the sn-1 and sn-3 positions. When these plants were crossed with transgenic lines expressing coconut LPAT, laurate was present at the sn-2 position along with the sn-1 and sn-3 positions in the resulting hybrids. An overall increase in the oil content was also observed.
When the yeast LPAT genes SLC1 and SLC1-1 (mutant form of yeast LPAT) were expressed in B. napus and Arabidopsis under the CaMV35S promoter, the triacylglycerols and very-long-chain fatty acid (VLCFA) contents were increased by 56% and 80%, respectively. In the transgenic plants, seed weight increased indicating at least a partial contribution from enhanced oil content. In the total oil content, 60% to 75% consisted of VLCFAs and 40% that of non-VLCFAs such as palmitate, oleate, linoleate and linolenate. No increase in total oil content was reported in coconut or meadowfoam LPAT-transformed rapeseed. This could be due to different regulatory properties of the plant and yeast LPAT enzymes. The plant LPAT genes have 62% amino acid identity among themselves whereas the yeast gene has 24% homology. In transgenic plants the high expression of SLC1-1 gene did not correlate with an increased oil content indicating that even small levels of expression were sufficient to overcome the phosphatidic acid limitations during triacylglycerol biosynthesis. Although SLC1-1 levels were higher in leaves than in seeds, no significant changes were observed in the fatty acid composition of leaves indicating the pools of available lysophosphatidic acid and/or acyl-CoAs may be more tightly regulated in leaves (source) than in seeds (sink).
By comparing the predicted amino acid sequence of the cat's claw (which accumulates nearly 80% palmitoleic acid (16:1∆9) plus cis-vaccenic acid (18:1∆11) in its seed oil) ∆9-18:0-ACP desaturase enzyme with that of the castor enzyme, it has been suggested that a single amino acid substitution (L118W) might account in large part for the differences in substrate specificity between the two desaturases. By converting leucine-118 to tryptophan in the mature castor ∆9-18:0-ACP desaturase, it was possible to increase the relative specificity of this enzyme for 16:0-ACP by 80-fold. As the amino acid sequences of all of the acyl-ACP desaturases are highly homologous and are colinear over most of their length, these researchers targeted the eight residues located near the bottom of the substrate-binding pocket that help to determine the chain-length specificity of the enzymes. The first of these residues, M114 of the mature castor protein, corresponds to M167 of the cat's claw sequence and M133 of the milkweed (which also has seeds enriched in palmitoleic (16:1∆9) and cis-vaccenic (18:1∆11) acids). Out of the eight residues, three are altered in the milkweed enzyme relative to the castor sequence (L115I, T117R, and P179T) but only one change is observed in the cat's claw protein L118W. Because of its bulky side chain, tryptophan 118 can be predicted to reduce the depth of the substrate pocket and thus favor the binding of 16:0-ACP in comparison to 18:0-ACP. An engineered L118W variant of the ∆9-18:0-ACP castor desaturase resulted in 115% activity with 16:0-ACP relative to its activity with 18:0-ACP. Similarly when the ORF of G188L mutant was linked to the plastid transit peptide of the coriander ∆4-16:0-ACP desaturase and expressed in A. thaliana under the napin promoter, single seeds from transgenic T1 plants accumulated 16:1∆9, 18:1∆11, and 20:1∆13 to amounts as high as 13% of the total fatty acids of the seed oil. In contrast, these fatty acids accounted for 3-4% of the total fatty acids in seeds of wild-type Arabidopsis transformed with the expression vector alone or with the wild-type castor ∆9-18:0-ACP desaturase.
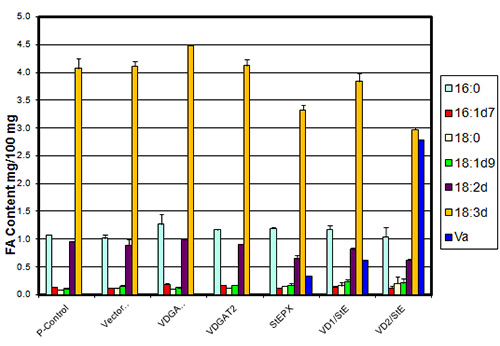
In studies on the expression profiles of genes encoding triacylglycerol biosynthetic enzymes, it was found that DGAT1, unlike DGAT2 or PDAT, has an expression profile in different tissues of soybeans and Arabidopsis consistent with a role in seed oil synthesis. DGAT1 and DGAT2, in contrast, display expression consistent with a role in seed oil synthesis in high epoxy and hydroxy fatty acid-accumulating plants implicating DGATs, particularly DGAT2, as playing an important role in the selective accumulation of unusual fatty acids in triacylglycerols. It has been established that DGAT2 has no significant role in triacylglycerol biosynthesis in Arabidopsis. However, there is good evidence that DGAT2 from tung trees, Vernicia fordii, has specificity for the conjugated fatty acid that accumulates in tung oil, eleostearic acid. co-expression of hydroxylase and DGAT2 from castor and epoxygenase + DGAT2 from Bernardia and Vernonia can increase the accumulation of hydroxy and epoxy fatty acids in seed oil up to fivefold over expression of the hydroxylase and epoxygenase genes alone (Fig. 3).
Figure 3. Epoxy fatty acid biosynthesis and accumulation gene tests in petunia leaves. Va = the epoxy fatty acid vernolic acid; P-Control = untransfected petunia leaves; Vector = vector control; VDGAT1a = expressing DGAT1a from Vernonia galamensis; VDGAT2 = expressing DGAT2 from V. galamensis; StEPX = expressing a Stokesia laevis epoxygenase; VD1/StE & VD2/StE = DGAT + epoxygenase.
This has been demonstrated in a commercial oilseed going from about 5% epoxy fatty acid in seed lipids of soybeans expressing an epoxygenase, increasing to >10% in soybeans expressing an epoxygenase + a DGAT1 from a high epoxy fatty acid-accumulating plant and to ~30% in soybeans expressing an epoxygenase + a DGAT2 from the same high epoxy fatty acid -ccumulating plant.
Perspective
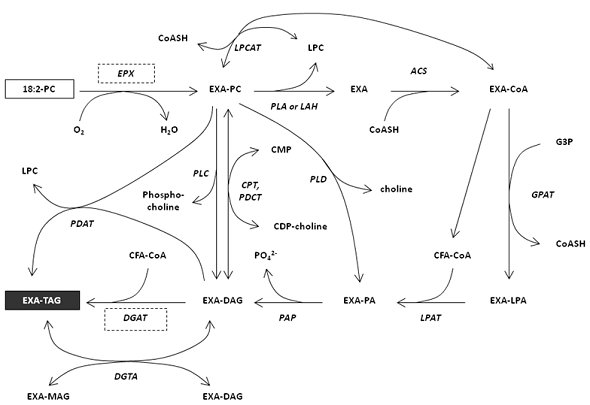
Possible routes for the biosynthesis of triacylglycerols in an epoxy fatty acid accumulating-plant such as Vernonia from the initial biosynthesis on phosphatidylcholine to the accumulation in triacylglycerols are illustrated in Figure 4.
Figure 4. Biosynthesis of triacylglycerol (TAG)-containing epoxy fatty acids (EXA)
ACS, acyl-CoA synthetase; EXA, epoxy fatty acid; EPX, epoxygenase; CoA, coenzyme A; CoASH, coenzyme A, free form; CDP, cytidine 5'-diphosphate; CMP, cytidine 5'-monophosphate; CPT, diacylglycerol cholinephosphotransferase; DAG, diacylglycerol; DGAT, acyl-CoA:diacylglycerol acyltransferase; DGTA, diacylglycerol:diacylglycerol transacylase; G3P, glycerol 3-phosphate; GPAT, glycerol 3-phsophate acyltransferase; LAH, lipolytic acyl hydrolase; LPA, lysophosphatidic acid; LPAT (LPAAT), lysophosphatidic acid acyltransferase; LPC, lysophosphatidyl choline; LPCAT, lysophosphatidyl choline acyltransferase; MAG, monoacylglycerol; PA, phosphatidic acid; PAP, phosphatidate phosphatase; PC, phosphatidyl choline; PDAT, phospholipid: diacylglycerol acyltransferase; PDCT, phosphatidylcholine:diacylglycerol cholinephosphotransferase; PLA, phospholipase A; PLC, phospholipase C; PLD, phospholipase D; TAG, triacylglycerol; UFA, unsaturated fatty acid
As of October 2010, we only have good information on the first and last steps of this biosynthetic pathway and much of the intermediate steps remains to be elucidated. Co-expression of genes encoding intermediate enzymes involved in the selective accumulation of unusual fatty acids in triacylglycerols should allow high economical production of unusual fatty acids in oilseeds as a renewable chemical source.
Acknowledgements
Related research in the author’s lab has been supported by United Soybean Board, Ashland Chemicals, Kentucky Science & Engineering Foundation and the Consortium for Plant Biotechnology Research.
Some Key References
- Bafor, M., Smith, M.A, Jonsson, L., Stobart, K. and Stymme, S. Biosynthesis of vernoleate (cis-12-epoxyoctadeca-cis-9-enoate) in microsomal preparations from developing endosperm of Euphorbia lagascae. Arch. Biochem. Biophys., 303, 145-151 (1993).
- Burgal, J., Shockey, J., Lu, C.F., Dyer, J., Larson, T., Graham, I. and Browse, J. Metabolic engineering of hydroxy fatty acid production in plants: RcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol. J., 6, 819-831 (2008) (DOI: 10.1111/j.1467-7652.2008.00361.x).
- Cahoon, E.B, Shah, S., Shanklin, J. and Browse, J. A determinant of substrate specificity predicted from the acyl-acyl carrier protein desaturase of developing cat's claw seed. Plant Physiol., 117, 593-598 (1998).
- Cahoon, E.B, Ripp, K.G, Hall, S.E. and Kinney, A.J. Formation of conjugated Δ8,Δ10-double bonds by Δ12-oleic-acid desaturase-related enzymes. J. Biol. Chem., 276, 2637-2643 (2001).
- Cahoon, E.B, Ripp, K.G, Hall, S.E. and McGonigle, B. Transgenic production of epoxy fatty acids by expression of a cytochrome P450 enzyme from Euphorbia lagascae seed. Plant Physiol., 128, 615-624 (2002).
- Dyer, J. and Mullen, R.T. Engineering plant oils as high-value industrial feedstocks for biorefining: the need for underpinning cell biology research. Physiol. Plant., 132, 11-22 (2008).
- Eccleston, V.S. and Ohlrogge, J.B. Expression of lauroyl-acyl carrier protein thioesterase in Brassica napus seeds induces pathways for both fatty acid oxidation and biosynthesis and implies a set point for triacylglycerol accumulation. Plant Cell, 10, 613-622 (1998).
- Eckert, H., LaVallee, B., Schweiger, B.J., Kinney, A.J., Cahoon, E.B. and Clemente, T. Co-expression of the borage Δ6 desaturase and the Arabidopsis Δ15 desaturase results in high accumulation of stearidonic acid in the seeds of transgenic soybean. Planta, 224, 1050-1057 (2006).
- Knutzon, D.S., Lardizabal, K.D., Nelsen, J.S., Bleibaum, J.L., Davies, H.M. and Metz, J.G. Cloning of a coconut endosperm cDNA encoding a 1-acyl-sn-glycerol-3-phosphate acyltransferase that accepts medium-chain-length substrates. Plant Physiol., 109, 999–1006 (1995).
- Lee, M., Lenman, M., Bana, A., Bafor, M., Singh, S., Schweizer, M., Nilsson, R., Liljenberg, C., Dahlqvist, A., Gummeson, P.-O., Sjödahl, S., Green, A. and Stymne, S. Identification of non-heme diiron proteins that catalyze triple bond and epoxy group formation. Science, 280, 915-918 (1998).
- Li, R., Yu, K. and Hildebrand, D. DGAT1, DGAT2 and PDAT expression in seeds and other tissues of epoxy and hydroxy fatty acid accumulating plants. Lipids, 45, 145-157 (2010).
- Li, R., Yu, K, Hatanaka, T. and Hildebrand, D.F. Vernonia DGATs increase accumulation of epoxy fatty acids in oil. Plant Biotechnol. J., 8, 184-195 (2010) (DOI: 10.1111/j.1467-7652.2009.00476.x).
- Liu, W., Torisky, R.S., McAllister, K.P., Avdiushko, S., Hildebrand, D. and Collins, G.B. A mammalian desaturase gene lowers saturated fatty acid levels in transgenic soybean embryos. Plant Cell Tissue Organ Culture, 47, 33-42 (1996).
- Millar, A.A, Smith, M.A. and Kunst, L. All fatty acids are not equal: discrimination in plant membrane lipids. Trends Plant Sci., 5, 95-101 (2000).
- Mongrand, S., Bessoule, J.-J., Cabantous, F. and Cassagne, C. The C16:3/C18:3 fatty acid balance in photosynthetic tissues from 468 plant species. Phytochemistry, 49, 1049-1064 (1998).
- Napier, J.A. and Graham, I.A. Tailoring plant lipid composition: designer oilseeds come of age. Curr. Opin. Plant Biol., 13, 330-337 (2010) (DOI: 10.1016/j.pbi.2010.01.008).
- Ruiz-López, N., Haslam, R.P., Venegas-Calerón, M., Larson, T.R., Graham, I.A., Napier, J.A. and Sayanova, O. The synthesis and accumulation of stearidonic acid in transgenic plants: a novel source of 'heart-healthy' omega-3 fatty acids. Plant Biotechnol. J., 7, 704-716 (2009).
- Shanklin, J. and Cahoon, E.B. Desaturation and related modifications of fatty acids. Annu. Rev. Plant Physiol. Plant Mol. Biol., 49, 611-641 (1998).
- Shockey, J.M., Gidda, S.K., Chapital, D.C., Kuan, J.-C., Dhanoa, P.K., Bland, J.M., Rothstein, S.J., Mullen, R.T. and Dyer, J.M. Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell, 18, 2294-2313 (2006).
- Slack, C.R., Roughan, P.G. and Balasingham, N. Labelling of glycerolipids in the cotyledons of developing oilseeds by [1-14C] acetate and [2-3H] glycerol. Biochem. J., 170, 421-433 (1978).
- Somerville, C., Browse, J., Jaworski, J.G. and Ohlrogge, J.B. Lipids. In: Biochemistry and Molecular Biology of Plants. pp. 456-527 (Eds. B.B. Buchanan, et al., American Society of Plant Physiologists, Rockville, Maryland) (2000).
- van de Loo, F.J., Fox, B.G. and Somerville, C. Unusual fatty acids. In: Lipid Metabolism in Plants. pp. 91-126 (Ed. J.T.S. Moore, CRC Press, Boca Raton) (1993).
- van de Loo, F.J, Broun, P., Turner, S. and Somerville, C. An oleate 12-hydroxylase from Ricinus communis L. is a fatty acyl desaturase homolog. Proc. Natl. Acad. Sci. U.S.A., 92, 6743-6747 (1995).
- Voelker, T. and Kinney, A.J. Variations in the biosynthesis of seed-storage lipids. Annu. Rev. Plant Physiol. Plant Mol. Biol., 52, 335-361 (2001).
- Xu, J., Francis, T., Mietkiewska, E., Giblin, E.M., Barton, D.L., Zhang, Y., Zhang, M. and Taylor, D.C. Cloning and characterization of an acyl-CoA-dependent diacylglycerol acyltransferase 1 (DGAT1) gene from Tropaeolum majus, and a study of the functional motifs of the DGAT protein using site-directed mutagenesis to modify enzyme activity and oil content. Plant Biotechnol., J. 6, 799-818 (2008).
- Zhang, M., Fan, J., Taylor, D.C. and Ohlrogge, J.B. DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell, 21, 3885-3901 (2009).
In This Section
- Plant Fatty Acid Synthesis
- Production of Unusual Fatty Acids in Plants
- Arabidopsis Acyl-Coenzyme A-Binding Proteins
- Long Chain acyl-coA Synthetases and Other Acyl Activating Enzymes
- Plant Triacylglycerol Synthesis
- Triacylglycerol Biosynthesis in Eukaryotic Microalgae
- Subcellular Oil Droplets and Oleosins in Plants
- Triacylglycerol Mobilisation in Plants
- Role of Transcription Factors in Storage Lipid Accumulation in Plants
- Biosynthesis of Plant Lipid Polyesters
- Rubber Biosynthesis in Plants
- Carotenoid Biosynthesis and Regulation in Plants
- The Oxylipin Biosynthetic Pathways in Plants
- N-Acylphosphatidylethanolamines (NAPEs), N-acylethanolamines (NAEs) and Other Acylamides: Metabolism, Occurrence and Functions in Plants
- Phosphoinositide Signaling in Plants
- Plant Lipidomics
- 50 years of Galactolipid Research: The Beginnings
- Transport and function of lipids in the plant phloem