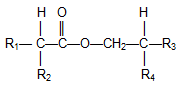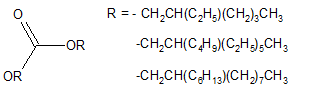Guerbet Compounds
The Author: Gerhard H. Knothe
Synthesis
The Guerbet reaction is named after Marcel Guerbet (1861-1938) [1]. However, it is unclear if Guerbet was indeed the first to discover the reaction, because Markovnikov (Markownikoff) stated that he made a similar discovery earlier [2].
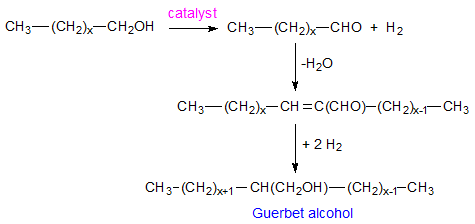
The Guerbet reaction is summarily a dimerization of alcohols with liberation of water to give branched alcohols with twice the number of carbons as the starting material (see overall scheme in Fig. 1).
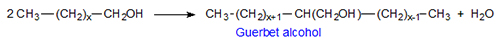
Figure 1. Overall Guerbet reaction.
These “typical” Guerbet alcohols have an even number of carbons with a minimum of six carbon atoms. The number of carbons in the main chain is always greater by four than that of the side chain. The Guerbet reaction of C16-18 fatty alcohols was described in publications dating from the 1950s [3,4]. A list of typical branched Guerbet compounds [5] and some of their properties is given in Table 1. As discussed here, there is significant commercial interest in compounds of the Guerbet kind as they posses good lubricity and a high fluidity range determined by low melting point and high boiling point. A functional group such as OH can impart a higher boiling point while branching of a hydrocarbon chain can lead to lowering of the melting point.
| Table 1. Guerbet alcohols (adapted from Ref. [5]). | ||||||||||
| Compound | Formula | Chem. Abstr. Reg. No. |
Molecular weight |
Melting point (°C) | Boiling point (°C) | |||||
| 2-Methyl-1-pentanol | C6H14O | 105-30-6 | 102.177 | 142-144 / 148 /149 (88 HCP) | ||||||
| 2-Ethyl-1-hexanol | C8H18O | 104-76-7 | 130.231 | -70 | 184.6 | |||||
| 2-Propyl-1-heptanol | C10H22O | 10042-59-8 | 158.285 | 217.5 | ||||||
| 2-Butyl-1-octanol | C12H26O | 3913-02-8 | 186.339 | 131-13312 / (HCP 88) | ||||||
| 2-Pentyl-1-nonanol | C14H30O | 5333-48-2 | 214.393 | 105-1060.6 / 16018 | ||||||
| 2-Hexyl-1-decanol | C16H34O | 2425-77-6 | 242.447 | -26 to -30 | 1220.5 / 181-18315 | |||||
| 2-Heptyl-1-undecanol | C18H38O | 5333-44-8 | 270.501 | 164-1663 / 19815 | ||||||
| 2-Octyl-1-dodecanol | C20H42O | 5333-42-6 | 298.555 | 160-1610.3 / 21515 | ||||||
| 2-Nonyl-1-tridecanol | C22H46O | 54439-52-0 | 326.609 | |||||||
| 2-Decyl-1-tetradecanol | C24H50O | 58670-89-6 | 354.663 | 175-1770.6 / 25015 | ||||||
| 2-Undecyl-1-pentadecanol | C26H54O | 79864-02-1 | 382.717 | |||||||
| 2-Dodecyl-1-hexadecanol | C28H58O | 72388-18-2 | 410.771 | |||||||
| 2-Tridecyl-1-heptadecanol | C30H62O | 183747-97-9 | 438.825 | |||||||
| 2-Tetradecyl-1-octadecanol | C32H66O | 32582-32-4 | 466.879 | 36.2 / 38-39 | 308-31015 | |||||
| 2-Pentadecyl-1-nonadecanol | C34H70O | 183747-96-8 | 494.933 | |||||||
| 2-Hexadecyl-1-eicosanol | C36H74O | 17658-63-8 | 522.987 | 43-45 | ||||||
| 2-Heptadecyl-1-heneicosanol | C38H78O | 181285-21-2 | 551.041 | |||||||
| 2-Octadecyl-1-docosanol | C40H82O | 182176-42-7 | 579.095 | |||||||
| 2-Nonadecyl-1-tricosanol | C42H86O | --- | 607.149 | |||||||
| 2-Eicosyl-1-tetracosanol | C44H90O | 73761-81-6 | 635.203 | |||||||
The Guerbet reaction occurs in the presence of an alkaline catalyst such as potassium hydroxide. Usually, transition metal-containing (Ni, Pd, Cu, Rh, Ir, etc.) components are added to the reaction system to catalyze hydrogenation, which can also lead to milder reaction conditions as the reaction is usually conducted at elevated temperatures under pressure. Figure 2 summarizes the reaction mechanism. Research has shown that reaction occurs in several steps [6-8] which are: (i) dehydrogenation of the alcohol which can also be catalyzed by the transition metal, (ii) aldol condensation of the carbonyl compounds formed in the first step, followed by liberation of water, (iii) hydrogenation of the α,β-unsaturated ketone formed in the previous step with the alcohol being the hydrogen donor, and (iv) disproportionation of aldehyde to alcohol and acid which terminates the chain reaction initiated in the previous step. More details on the mechanism are given in the literature [6-8 and references therein].
Figure 2. Reaction steps of the Guerbet reaction.
Some examples of Guerbet reactions are the condensation of ethanol or 1-butanol to 1-butanol or 2-ethyl-1-hexanol under more mildly basic conditions than normally employed in the Guerbet reaction was reported with an MgO, K2CO3, Cu2Cr2O5 (copper chromite) catalyst system leading to higher alcohols with a negligible amount of carboxylic acid by-product [9]. The observation was consistent with those of other researchers who have pointed out the necessity for an alcohol to have two β-hydrogens in order to participate in a Guerbet dimerization reaction. A modified Guerbet reaction with benzalaniline and U.O.P. nickel afforded Guerbet products with normal primary alcohols with benzyl alcohol also condensing with these alcohols and cyclohexanol condensing with either [10]. More recently, ethanol was converted to butanol with [Ir(acac)(cod)], 1,7-octadiene, and EtONa with this process stated to be a possible alternative to conventional butanol syntheses [11]. Hydroxyapatite catalysts converted ethanol into a variety of Guerbet alcohols during the synthesis of what the authors termed “biogasoline” from ethanol [12]. Relatedly, [IrCl(cod)]2 and [Cp*IrCl2]2 catalyzed the Guerbet reaction of primary alcohols to β-alkylated primary alcohols, an example being the synthesis of 2-ethyl-1-hexanol from butanol [13]. A review on the condensation of low-molecular weight alcohols (methanol, ethanol) into higher alcohols (butanol and higher) is Ref. [14].
Alcohols other than the typical ones shown in Figures 1 and 2 and listed in Table 1 can be obtained from a Guerbet reaction. Secondary alcohols have been condensed under Guerbet conditions [8]. Several cross-condensations of mixtures of different alcohols subjected to Guerbet reactions have been reported. These include: (i) reaction of methanol with 1-butanol or 1-pentanol in presence of a rhodium catalyst [8]; (ii) synthesis of 1-propanol and 2-methyl-1-propanol from ethanol and methanol using magnesium oxide [15]; (iii) synthesis of iso-butanol from methanol and n-propanol using various metal-based catalysts [16-20] and a related system for the condensation of methanol and ethanol or methanol, ethanol, and n-propanol [21]; and (iv) alkylation of cyclic secondary alcohols with primary alcohols for the combinations cyclopentanol/1-heptanol and cyclohexanol/2-ethyl-1-hexanol with side products arising in varying amounts depending on reaction conditions such as molar ratio [22]. Reactions of fatty alcohols of chain length C8-C14 and benzyl alcohol yielded 2-benzyl fatty alcohols [23]. A Guerbet reaction of isopropanol to give 3,3,5-trimethylcyclohexanol was observed in a study of the decomposition of epoxy resin with KOH in supercritical isopropanol [24]. Other classes of compounds can be subjected to reactions under Guerbet conditions. For example, amines reacted under Guerbet conditions gave imines as major product [25].
Other Guerbet-type compounds. Guerbet alcohols can be used to prepare other derivatives. A straightforward example is Guerbet acids obtained by oxidation of Guerbet alcohols, for example using Pd, Pt, or Ru catalysts [26]. Guerbet alcohols also can be esterified with a variety of carboxylic acids such as fatty or Guerbet acids, the latter ester species being termed di-Guerbet esters [27] shown in Figure 3.
Figure 3. Di-Guerbet esters.
Carbonates synthesized from Guerbet alcohols [28,29] depicted in Figure 4 and esters of fatty and dicarboxylic acids with Guerbet alcohols [30] have also been reported.
Figure 4. Guerbet carbonates.
The patent literature, a list of some patents through 2002 being given in Ref. [31], provides information on various derivatives of Guerbet compounds, including alkoxylated Guerbet alcohols and esters, Guerbet amines, betaines, branched amine oxides, carbonates, esters of meadowfoam oil and ricinoleic acid (castor oil), fluorinated citrate esters, lactams, polyoxyalkylene glycol and sorbitan esters, etc.
Properties and Applications
As indicated above, their wide liquidity range, documented by melting (especially) and boiling points, is one reason why Guerbet compounds have significant practical applications. For example, 1-octanol has a melting point of -16.7°C and a boiling point of 194.4°C. The Guerbet alcohol with the same number of carbon atoms, 2-ethyl-1-hexanol, has a melting point of -70°C and a boiling point of 184.6°C (Table 1).
Other properties that make Guerbet compounds of interest are good lubricity, good oxidative stability since they are saturated compounds, and volatility comparable to the straight-chain isomers (the boiling points are only slightly reduced compared to the straight-chain congeners, see the above example).
Numerous patents describe the uses of Guerbet compounds and their derivatives. The list in the literature [31] also provides related information. The applications for C12-36 Guerbet alcohols may be summarized as components of cosmetics and lubricants as well as solvents or solubilizers for printing colors and inks and starting materials for other materials with other applications including surfactants. Higher Guerbet alcohols (C32-36) are useful for the production of specialty waxes and cosmetic uses. Related materials such as Guerbet acids, or esters of other acids with Guerbet alcohols, are also of commercial interest and they have similar applications. The benzyl fatty alcohols are useful as surfactants as are Guerbet sulfates and ether sulfates [31].
Citrate esters (R-CO-CH2-C(OH)(COR)-CH2-COR, R derived from a Guerbet alcohol) provide non-irritating high-cushion emolliency, while fluoro-Guerbet citrates (R-CO-CH2-C(OH)(COR)-CH2-COR´ with R´ = CF3-(CF2)n-CH2-CH2-O-) provide breathable, nonocclusive films [32].
Numerous publications deal with the surface properties of Guerbet and related compounds. Relevant properties, such as surface tension and contact angle important for surfactant applications, are influenced by structural features such as the position of the OH group, location of branching, and length of side chains besides overall molecular weight [33-38]. Guerbet alcohol sodium sulfates can be components of microemulsions [39]. Sulfated Guerbet alcohol ethoxylates and propoxylates [40,41] as well as Guerbet alcohol hexadecyl glycidyl ether ammonium chloride [42] were also applied in surfactant systems and again structural effects were observed. N-Methyl-N-D-gluca-2-butyloctanamide was investigated as a nonionic surfactant yielding aqueous microemulsions without electrolyte [43].
2-Ethyl-1-hexanol. The derivatization of a Guerbet compound with other compounds and the resulting expansion of utility is shown by the probably best-known representative of the typical Guerbet alcohols, 2-ethyl-1-hexanol. With a production volume of about 2 x 106 t/a a few years ago, it is the most important industrial alcohol after the lighter C1-4 alcohols [44]. For economic reasons, however, it is produced industrially not from butanol but from butanal which in turn is derived from propene [44]. The main use (>60%) of 2-ethylhexanol is the production of plasticizers such as diethylhexyl phthalate (DEHP; dioctyl phthalate) and diethylhexyl adipate [44]. Other uses of 2-ethylhexanol include the production of 2-ethylhexyl acrylate, which in turn is used in coating materials, adhesives, and inks, 2-ethylhexylnitrate used as cetane improver additives for diesel fuel, and 2-ethylhexylphosphates as an additive for lubricating oils [44].
An alternative synthesis of the corresponding acid, 2-ethylhexanoic acid, is achieved by hydrogenating the δ-lactone depicted in Figure 5 in the presence of a water-soluble rhodium-phosphine catalyst [45]. The δ-lactone is obtained from a reaction of butadiene with carbon dioxide.
Figure 5. δ-Lactone from butadiene and CO2 for synthesis of 2-ethylhexanoic acid.
References
- Guerbet, M. Action de l’alcool amylique de fermentation dérivé sodé. Comptes rendus, 128, 511-513 (1899).
- Markownikoff, W. and Zuboff, P. Ueber die condensation höherer alkohole: tricaprylalkohol. Chem. Ber., 34, 3246-3249 (1901).
- Sulzbacher, M. The Guerbet reaction of cetyl alcohol. J. Appl. Chem., 5, 637-641 (1955).
- Gast, L.E., Bitner, E.D., Cowan, J.C. and Teeter, H.M. Reactions of unsaturated fatty alcohols. VI. Guerbet reaction of soybean and linseed alcohols. J. Am. Oil Chem. Soc., 35, 703-707 (1958).
- Knothe, G. Synthesis, applications, and characterization of Guerbet compounds and their derivatives. Lipid Technol., 14, 101-104 (2002).
- Veibel, S., and Nielsen, J.I. On the mechanism of the Guerbet reaction. Tetrahedron, 23, 1723-1733 (1967).
- Burk, P.L., Pruett, R.L, and Campo, K.S. The rhodium-promoted Guerbet reaction. Part I. Higher alcohols from lower alcohols. J. Mol. Catal., 33, 1-14 (1985).
- Burk, P.L., Pruett, R.L, and Campo, K.S. The rhodium-promoted Guerbet reaction. Part II. Secondary alcohols and methanol as substrates. J. Mol. Catal., 33, 15-21 (1985).
- Dvornikoff, M.N. and Farrar, M.W. Condensation of alcohols. J. Org. Chem., 22, 540-542 (1957).
- Pratt, E.F., and Kubler, D.G. Disproportionative condensations. I. Modified Guerbet reactions. J. Am. Chem. Soc., 76, 52-56 (1954).
- Koda, K. , Matsu-ura, T., Obora, Y. and Ishii, Y. Guerbet reaction of ethanol to n-butanol catalyzed by iridium complexes. Chem. Lett., 38, 838-839 (2009).
- Tsuchida, T., Yoshioka, T., Sakuma, S., Takeguchi, T., and Ueda, W. Synthesis of biogasoline from ethanol over hydroxyapatite catalyst. Ind. Eng. Chem. Res., 47, 1443-1452 (2008).
- Matsu-ura, T., Sakaguchi S., Obora, Y. and Ishii, Y. Guerbet reaction of primary alcohols leading to β-alkylated dimer alcohols catalyzed by iridium complexes. J. Org. Chem., 71, 8306-8308 (2006).
- Olson, E.S., Sharma, R.K. and Aulich, T.R. Higher-alcohols biorefinery: improvement of catalyst for ethanol conversion. Appl. Biochem. Biotech., 115, 913-932 (2004).
- Ueda, W., Kuwabara, T., Oshida, T. and Morikawa, Y. A low-pressure Guerbet reaction over magnesium oxide catalyst. J. Chem. Soc., Chem. Commun., 1558-1559 (1990).
- Carlini, C., Di Girolamo, M., Marchionna, M., Noviello, M., Galletti, A.M.R. and Sbrana, G. Selective synthesis of isobutanol by means of the Guerbet reaction. Part 1. Methanol/n-propanol condensation by using copper based catalytic systems. J. Mol. Catal. A: Chem., 184, 273-280 (2002).
- Carlini, C., Macinai, M., Marchionna, M., Noviello, M., Galletti, A.M.R. and Sbrana, G. Selective synthesis of isobutanol by means of the Guerbet reaction. Part 3. Methanol/n-propanol condensation by using bifunctional catalytic systems based on nickel, rhodium, and ruthenium species with basic components. J. Molec. Catal. A: Chem., 206, 409-418 (2003).
- Carlini, C., Di Girolamo, M., Macinai, M., Marchionna, M., Noviello, M., Galletti, A.M.R., and Sbrana, G. Selective synthesis of isobutanol by means of the Guerbet condensation of methanol with n-propanol in the presence of heterogeneous and homogeneous palladium-based catalytic systems. J. Molec. Catal. A: Chem., 204-5, 721-728 (2003).
- Carlini, C., Flego, C., Marchionna, M., Noviello, M., Galletti, A.M.R., Sbrana, G., Basile, F. and Vaccari, A. Guerbet condensation of methanol with n-propanol to isobutyl alcohol over heterogeneous copper chromite/Mg-Al mixed oxides catalysts. J. Molec. Catal. A: Chem., 220, 215-220 (2004).
- Carlini, C., Marchionna, M., Noviello, M., Galletti, A.M.R., Sbrana, G., Basile, F., and Vaccari, A. Guerbet condensation of methanol with n-propanol to isobutyl alcohol over heterogeneous bifunctional catalysts based on Mg-Al mixed oxides partially substituted by different metal components. J. Molec. Catal. A: Chem., 232, 13-20 (2005).
- Carlini, C., Di Girolamo, M., Macinai, M., Marchionna, M., Noviello, M. Galletti, A.M.R., and Sbrana, G. Selective synthesis of isobutanol by means of the Guerbet reaction. Part 2. Reaction of methanol/ethanol and methanol/ethanol/n-propanol mixtures over copper based/MeONa catalytic systems. J. Molec. Catal. A: Chem., 200, 137-146 (2003).
- Schaper, U.-A. Die gemischte Guerbet-Reaktion zwischen cyclischen und acyclischen alkoholen. Fette, Seifen, Anstrichm., 82, 454-456 (1980).
- Krause, H.-J. and Syldatk, A. Neue tenside aus gemischten Guerbet-alkoholen. Fette, Seifen, Anstrichm., 87, 386-390 (1985).
- Jiang, G., Pickering, S.J., Lester, E.H., and Warrior, N.A. Decomposition of epoxy resin in supercritical isopropanol. Ind. Eng. Chem. Res., 49, 4535-4541 (2010).
- Miller, R.E. The Guerbet reaction. I. The reaction of amines under Guerbet conditions. J. Org. Chem., 25, 2126-2128 (1960).
- Behr, A. and Döring, N. Synthesis of branched fatty acids by catalytic oxidation of alcohols. (Herstellung verzweigter fettsäuren durch katalytische oxidation von alkoholen.). Fat Sci. Technol., 94, 13-18 (1992).
- Knothe, G. and Carlson, K.D. Synthesis, mass spectrometry, and nuclear magnetic resonance characterization of di Guerbet esters. J. Am. Oil Chem. Soc., 75, 1861-1866 (1998).
- Kenar, J.A., Knothe, G. and Copes, A.L. Synthesis and characterization of dialkyl carbonates prepared from mid-, long-chain, and guerbet alcohols. J. Am. Oil Chem. Soc., 81, 285–291 (2004).
- Kenar, J.A., Knothe, G., Dunn, R.O., Ryan, T.W. and Matheaus, A. Physical properties of oleochemical carbonates. J. Am. Oil Chem. Soc., 82, 201–205 (2005).
- Knothe, G. Characterization of esters of fatty acids and dicarboxylic acids with Guerbet alcohols. J. Am. Oil Chem. Soc., 78, 537–540 (2001).
- O’Lenick, A.J. Guerbet chemistry. J. Surfact. Deterg., 4, 311-315 (2001).
- O'Lenick, A.J., Jr., Parkinson, J.K. and Buffa, C.W. Guerbet citrate esters. Cosmetics & Toiletries, 110, 73-77 (1995).
- Finger, B.M., Gillies, G.A., Hartwig, G.M., Ryder, E.E., Jr. and Sawyer, W.M. Detergent alcohols. I. The effect of alcohol structure and molecular weight on surfactant properties. J. Am. Oil Chem. Soc., 44, 525-530 (1967).
- O'Lenick, A.J., Jr. and Parkinson, J.K. Effects of branching upon some surfactant properties of sulfated alcohols. J. Am. Oil Chem. Soc., 73, 935-937 (1996).
- Varadaraj, R., Bock, J., Valint, P., Jr., Zushma, S. and Thomas, R. Fundamental interfacial properties of alkyl-branched sulfate and ethoxy sulfate surfactants derived from Guerbet alcohols. 1. Surface and interfacial tensions. J. Phys. Chem., 95, 1671-1676 (1991).
- Varadaraj, R., Bock, J., Valint, P., Jr., Zushma, S. and Brons, N. Fundamental interfacial properties of alkyl-branched sulfate and ethoxy sulfate surfactants derived from Guerbet alcohols. 2. Dynamic surface tensions. J. Phys. Chem., 95, 1677-1679 (1991).
- Varadaraj, R., Bock, J., Valint, P., Jr., Zushma, S. and Brons, N. Fundamental interfacial properties of alkyl-branched sulfate and ethoxy sulfate surfactants derived from Guerbet alcohols. 3. Dynamic contact angle and adhesion tension. J. Phys. Chem., 95, 1679-1681 (1991).
- Varadaraj, R., Bock, J., Valint, P., Jr. and Zushma, S. Thermodynamics of adsorption and micellization in linear and guerbet sulfate and ethoxy sulfate surfactants. J. Phys. Chem., 95, 1682-1684 (1991).
- Aoudia, M., Wade, W.H. and Weerasooriya, V. Optimum microemulsions formulated with propoxylated guerbet alcohol and propoxylated tridecyl alcohol sodium sulfates. J. Dispers. Sci. Technol., 16, 115-135 (1995).
- Baran, J.R., Jr., Pope, G.A., Wade, W.H. and Weerasooriya, V. Phase behavior of water/perchloroethylene/anionic surfactant systems. Langmuir, 10, 1146-1150 (1994).
- Oh, K.-H., Baran, J.R., Jr., Wade, W.H. and Weerasooriya, V. Temperature insensitive microemulsion phase behavior with non ionic surfactants. J. Dispers. Sci. Technol., 16, 165-188 (1995).
- Zhang, L., Wang, Z.-L., Li, Z.-Q., Zhang, L., Xu, Z.-C., Zhao, S. and Yu, J.-Y. Wettability of a quartz surface in the presence of four cationic surfactants. Langmuir, 26, 18834-18840 (2010).
- Arenas, E., Baran, J.R., Jr., Pope, G.A., Wade, W.H. and Weerasooriya, V. Aqueous phase microemulsions employing N-methyl-N-D-glucalkanamide surfactants with chlorinated hydrocarbons. Langmuir, 12, 588-590 (1996).
- Bahrmann, H., Hahn, H.D. and Mayer, D. 2-Ethylhexanol. In: Ullmann’s Encyclopedia of Industrial Chemistry, 6th Ed. (Wiley-VCH, Weinheim, online version) (2001).
- Behr, A., and Brehme, V.A. Homogeneous and heterogeneous catalyzed three-step synthesis of 2-ethylheptanoic acid from carbon dioxide, butadiene and hydrogen. J. Molec. Catal. A, 187, 69-80 (2002).
December 22, 2011