Formation of Epoxy-, Keto- and Hydroxy-Fatty Acids
The Author M. Carmen Dobarganes Instituto de la Grasa (CSIC), Avda Padre Garcia Tejero 4, 41012 Seville, Spain
1. Introduction
Oxidized triacylglycerol monomers are major compounds formed at frying temperature, and are characterized by the presence of one or more oxygenated function in at least one of the fatty acyl chains of the triacylglycerols. They are stable final products resulting from the decomposition of primary oxidation compounds (hydroperoxides), which are unstable at frying temperature. The main functional groups present in frying oils correspond to epoxy, keto and hydroxy, which do not continue the oxidation reaction sequences. However, because of the presence of double bonds, the oxidized fatty acids can undergo further oxidation.
The interest in the study of this group of compounds is related to both the high amounts formed during frying and the implication of oxidized dietary fats and oils in the impairment of the nutritional and physiological properties. Thus, quantification of total polar compounds and their distribution in used frying fats and oils around the limit of rejection (25% polar compounds) has shown that the amount of oxidized triglyceride monomers is considerable, ranging from 5.9% to 9.4% expressed on fat or oil weight [1].
Also, oxidized dietary fats have been implicated in a myriad of diseases, although assigning risk for a specific disease to a particular dietary pattern is challenging because the intake of oxidized fats is unknown. Nevertheless, most of the oxidized fats in foods are expected to come from fats and oils heated at high temperature and more specifically from frying fats [2]. The main reason is that at low temperature during oil storage, rarely more than 4 to 5% of triacylglycerols (around 100 meq O2/kg fat, a level at which rancid odour is clearly detectable) would be oxidized in the ingested foods as compared to the upper limit (25%) allowed in used frying fats, often surpassed in a significant number of oils and fats from fast-food outlets [3].
The main nutritional interest on oxidized monomers is supported by the high absorption of oxidized fatty acyl groups, considering that they have greater polarity and do not differ much in molecular weight when compared to unchanged fatty acids. Recently, the absorption of dietary labelled hydroxy and epoxy fatty acids incorporated in triacylglycerols was observed in humans [4,5], and special emphasis has been placed on hydroxy fatty acids, since not only are they present as stable compounds in the diet but they can also be formed after ingestion by reduction of hydroperoxides.
2. Epoxy-Acids
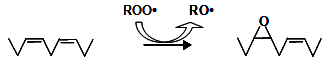
The formation of the epoxide ring has been reported to proceed through two distinct mechanisms at 50°C, either at the site of the double bond or nearby it [6]. In the latter case, the original double bond remains. However, only the compounds formed when the oxygen added across an existing double bond were detected at frying temperature [7-9]. Figure 1 shows the proposed mechanism consisting of a direct attack by a hydroperoxyl radical on the vinylic carbons, leading to the formation of an oxirane group and an alkoxyl radical.
Figure 1. Mechanism involved in the formation of oxirane ring.
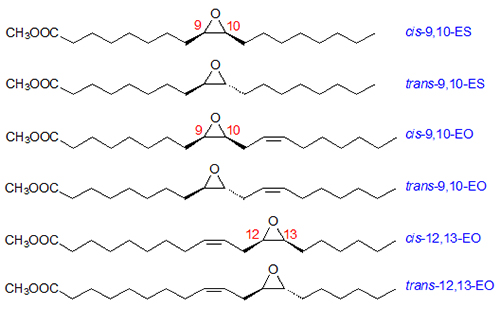
Figure 2 illustrates the main structures formed at high temperature from oleyl and linoleyl groups after derivatization to fatty acid methyl esters (FAME). Thus, two saturated epoxides, trans-9,10- and cis-9,10-epoxystearate (ES), were found in samples of methyl oleate and triolein thermoxidized at 180°C. In addition, four monounsaturated epoxides, trans-12,13-, trans-9,10-, cis-12,13- and cis-9,10-epoxyoleate (EO), were identified in thermoxidized samples of methyl linoleate and trilinolein [7]. Further results showed that the six epoxides coming from the oleyl and linoleyl chains make up a major group of oxidation compounds in used frying oils.
Figure 2. Monoepoxides obtained from oleic and linoleic acids.
3. Keto- and Hydroxy-Acids
Studies on formation of hydroxy- and keto-fatty acyl groups at frying temperature are scarce, as a high number of compounds are formed as compared to the epoxides. Most of the information has been obtained from fatty acids and FAME oxidized at low temperatures and has demonstrated that both groups of compounds are formed after decomposition of hydroperoxides.
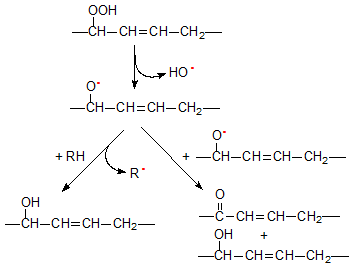
The most likely decomposition pathway of hydroperoxides is the homolytic cleavage of the hydroperoxide group to yield hydroxyl and alkoxyl radicals. The alkoxyl radical can participate in different reactions to produce compounds with keto and hydroxy functions as shown in a simplified manner in Figure 3. Hydroxy-acids may be formed through the abstraction of a hydrogen atom by an alkoxyl radical, while the formation of keto-acids has been explained on the basis of radical disproportionation where two alkoxyl radical combine to yield a hydroxy-acid and a keto-acid.
Figure 3. Formation of secondary oxidation products containing hydroxy and keto functions.
From oleic acid a mixture of the eight isomeric hydroperoxides are formed during oxidation, i.e. cis and trans 8-, 9-, 10- and 11 hydroperoxy octadecenoic acids, while from linoleic acid, a mixture of four major hydroperoxide isomers are formed, i.e. cis-9-trans-11 and trans-9-,trans-11 of 13-hydroperoxy-octadecadienoic acid and cis-10-,trans-11 and trans-10-,trans-12 of 9-hydroperoxy-octadecadienoic acid [11]. Thus, 24 individual compounds (12 hydroxy-acids and 12 keto-acids) are expected in used frying fats.
When FAME from used frying oils were analysed by GC-MS, keto- and hydroxy-FAME were found as numerous minor peaks that were not well resolved. They correspond, as expected, to diene keto- and hydroxy-FAME from linoleyl groups and monoene keto- and hydroxy-FAME from oleyl groups. However, the elution order of the unsaturated molecules was not that expected from the saturated compounds (saturated keto-FAME of lower polarity elute at lower retention time than saturated hydroxy-FAME). This fact is due to an increased polarity of keto-FAME provided by their conjugated triene or diene structure, i.e. conjugation of the diene structure with the carbonyl group, as compared to the conjugated diene structure present in hydroxy-FAME or conjugation of the monoene structure with the carbonyl group as compared with the hydroxy-monoenes.
Quantification of these groups of compounds has been possible after hydrogenation to modify the polarity of the compounds and to reduce the number of individual compounds. The levels of keto-FAME were significantly lower than those of hydroxy-FAME independently of the level of oil degradation, while hydroxy-FAME had similar levels to those found for epoxy-acids [12].
References
- Márquez-Ruiz, G., Tasioula-Margari, M. and Dobarganes, M.C. Quantitation and distribution of altered fatty acids in frying fats. J. Am. Oil Chem. Soc., 72, 1171-1176 (1995).
- Dobarganes, M.C. and Márquez-Ruiz, G. Oxidized fats in foods. Curr. Opin. Clin. Nutr. Metab. Care, 6, 157-163 (2003).
- Saguy, I.S. and Dana, D. Integrated approach to deep fat frying: engineering, nutrition, health and consumer aspects. J. Food Eng., 56, 143-152 (2003).
- Wilson, R., Lyall, K., Smyth, L., Fernie, C.E. and Riemersma, R.A. Dietary hydroxy fatty acids are absorbed in humans: implications for the measurement of 'oxidative stress' in vivo. Free Rad. Biol. Med., 32, 162-168 (2002).
- Wilson, R., Fernie, C.E., Scrimgeour, C.M., Lyall, K., Smyth, L. and Riemersma, R.A. Dietary epoxy fatty acids are absorbed in healthy women. Eur. J. Clin. Invest., 32, 79-83 (2002).
- Neff, W.E. and Byrdwell W.C. Characterization of model triacylglycerol (triolein, trilinolein and trilinolenin) autoxidation products via high-performance liquid chromatography coupled with atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A, 818,169-186 (1998).
- Berdeaux, O., Márquez-Ruiz, G. and Dobarganes, M.C. Characterization, quantitation and evolution af monoepoxy compounds formed in model systems of fatty acids methyl esters and monoacid triglycerides heated at high temperature. Grasas y Aceites, 50, 53-59 (1999).
- Lercker, G., Rodriguez-Estrada, M.T. and Bonoli, M. Analysis of the oxidation products of cis- and trans-octadecenoate methyl esters by capillary gas chromatography–ion-trap mass spectrometry. I. Epoxide and dimeric compounds. J. Chromatogr. A, 985, 333–342 (2003).
- Giuffrida, F., Destaillats, F., Robert, F., Skibsted, L.H. and Dionisi, F. Formation and hydrolysis of triacylglycerol and sterol epoxides: Role of unsaturated triacylglycerol peroxyl radicals. Free Rad. Biol. Med., 37, 104-114 (2004).
- Velasco, J., Marmesat, S., Bordeaux, O., Márquez-Ruiz, G. and Dobarganes, M.C. Formation and evolution of monoepoxy fatty acids in thermoxidized olive and sunflower oils and quantitation in used frying oils from restaurants and fried food outlets. J. Agric. Food Chem., 52, 4438- 4443 (2004).
- Porter, N.A., Caldwell, S.E. and Mills, K.A. Mechanisms of free radical oxidation of unsaturated lipids. Lipids, 30, 277-290 (1995).
- Marmesat, S., Velasco, J. and Dobarganes, M.C. Quantitative determination of epoxyacids, ketoacids and hydroxyacids formed in fats and oils at frying temperatures. J. Chromatogr. A, 1211, 129-134 (2008).
In This Section
- Oil Refining
- Action of Natural Antioxidants During Frying
- Formation of New Compounds During Frying - General Observations
- Formation of cyclic fatty acids during frying
- Formation of Epoxy-, Keto- and Hydroxy-Fatty Acids
- Formation of Volatiles and Short-Chain Bound Compounds
- Formation of Dimers and Oligomers
- Oxysterol Formation Frying Oils
- Structural Analysis of the Cyclic Fatty Acids Formed during Frying
- Cyclic Fatty Acids: Isolation and Quantitative Analysis in Food and Biological Tissues
- Analysis of Used Frying Oils and Fats by High-Performance Size-Exclusion Chromatography
- Analysis of Trans Polyunsaturated Fatty Acids
- Determination of Polar Compounds in Used Frying Oils and Fats by Adsorption Chromatography
- Determination of Oxidized Monomeric, Dimeric and Oligomeric Triacylglycerols; Diacylglycerols and Free Fatty Acids
- Separation and Quantification of Oxidized Monomeric, Dimeric and Oligomeric Fatty Acids
- Analysis of Oxidized Fatty Acids
- Analysis of Oxidized Sterols in Frying Oils
- Metabolism and Physiological Effects of Cyclic Fatty Acids Formed from Linoleic and alpha-Linolenic Acids during Frying
- Metabolism and Physiological Effects of Cyclic Fatty Acids Formed from Linoleic and alpha-Linolenic Acids during Frying
- Metabolism of Trans Polyunsaturated Fatty Acids Formed during Frying
- Biological Effects of Frying Oils Mediated by the Activation of Peroxisome Proliferator-Activated Receptors (PPAR)