Regulation of Lipins and Their Role in Lipid Metabolism
The Authors: Bernard P.C. Kok and David N. Brindley DOI: 10.21748/lipidlibrary.39189
The Role of Phosphatidate Phosphatase in Glycerolipid Biosynthesis
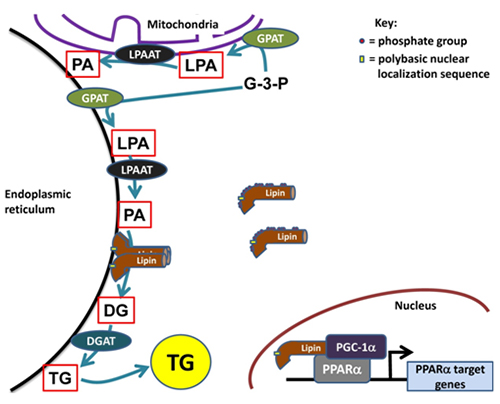
The glycerolipid biosynthetic or Kennedy pathway is responsible for the synthesis of triacylglycerol and phospholipids de novo. The entire pathway for triacylglycerol synthesis consists of four enzymatic steps, which catalyze sequential reactions starting from sn-glycerol-3-phosphate and ending with triacylglycerol (TG) (Fig. 1). Furthermore, two intermediates in this pathway, phosphatidic acid and diacylglycerol, serve as essential precursors for the synthesis of phospholipids. Phosphatidate can be converted to CDP-diacylglycerol which is the precursor for the acidic phospholipids, phosphatidylglycerol, cardiolipin and phosphatidylinositol, or it can be converted to diacylglycerol (DG) by dephosphorylation. Diacylglycerol is the direct precursor for the production of the zwitterionic phospholipids, phosphatidylcholine and phosphatidylethanolamine (Fig. 1). The enzyme that catalyzes dephosphorylation of phosphatidate is called phosphatidate phosphatase (PAP), or lipin, and this is an important branch point in glycerolipid biosynthesis.
Figure 1. The role of lipins in cellular metabolism. Lipins are cytosolic phosphatidate phosphatases (PAPs) involved in the glycerolipid biosynthesis pathway. This pathway begins with the acylation of glycerol-3-phosphate (G-3-P) by glycerol-3-phosphate acyltransferases (GPATs) at the sn-1 position followed by a second acylation of lysophosphatidate (LPA) at the sn-2 position by lysophosphatidate acyltransferases (LPAATs). The phosphatidate formed is dephosphorylated by the PAP activity of the lipins and lastly diacylglycerol acyltransferases (DGATs) act on diacylglycerol (DG) to form triacylglycerol (TG). Hyperphosphorylated lipins are relatively unable to translocate onto endoplasmic reticulum membranes. The polybasic nuclear localization sequence (NLS) on the lipins as well as the oligomerization of lipins mediates membrane association. Lipins also act in the nucleus as transcriptional co-activators with peroxisome proliferator-activated receptor γ co-activator-1α (PGC-1α) and peroxisome proliferator-activated receptor α to regulate target genes involved in fatty acid oxidation.
PAP controls the balance between phosphatidate, which is needed for the synthesis of acidic phospholipids, and diacylglycerol, which is used to synthesize triacylglycerols, phosphatidylcholine and phosphatidylethanolamine, respectively [1]. Despite the importance of the PAP reaction, identification of the genes responsible for encoding PAP enzymes remained elusive until 2006 when the yeast PAP was identified and was shown to be an orthologue of a family of three mammalian proteins called lipins [reviewed in 1]. All three mammalian lipins were subsequently shown to have PAP activity and to be expressed in a tissue-specific manner. For example, lipin-1 is the major PAP in adipose tissue, and cardiac and skeletal muscle since lipin-1-deficient mice had little if any detectable PAP activity [2]. Mice lacking lipin-1 (fld or fatty liver dystrophy) develop fatty livers and hypertriglyceridemia in the pre-weaning period, and are also devoid of mature adipose tissue [reviewed in 1]. Lipin-2 is important in the liver [4,5]. Lipin-3 is most highly expressed in the intestine as shown by the analysis of mRNA distribution in different tissues [4].
Lipin-1 is the best-characterized protein of the lipin family. The PAP activities of the lipins depend on a conserved C-terminus domain with sequence homology to the haloacid dehalogenase or HAD-like domain [1]. The DxDxT catalytic motif is present in all HAD-like domains, along with a characteristic and conserved sequence of α-helices and β-sheets [3]. The HAD domain-containing proteins catalyze hydrolase or phosphohydrolase reactions.
Mammalian Lipins Exhibit a Second Function as Co-transcriptional Regulators
Lipin-1 and -2 have a second function as transcriptional co-activators acting in a complex with transcriptional regulators called peroxisome proliferator-activated receptor-coactivator-1α (PGC-1α) and peroxisome proliferator-activated receptor-α (PPARα) [1,3] (Fig. 1). The co-transcriptional regulatory binding site on the lipins is a conserved LxxIL motif, which is distinct from the active site. Lipin-3 is also presumed to be able to act in a similar fashion through its LxxIL motif, but this has not yet been demonstrated experimentally. PGC-1α and PPARα are both master regulators of genes involved in mitochondrial biogenesis and fatty acid oxidation, respectively [reviewed in 6]. The functions of the lipins in co-regulating the effects of PGC-1α and PPARα and also acting as a PAP demonstrate the unique abilities of lipins to regulate both glycerolipid synthesis and fatty acid oxidation. This is an important finding since the modern view on these pathways is that triacylglycerol synthesis is a companion pathway that facilitates fatty acid oxidation. It is now thought that triacylglycerol turnover supplies a significant portion of the fatty acids that are subsequently oxidized [7]. Previously, it was considered that the branch point of fatty acid esterification and oxidation represented mutually antagonistic processes.
An additional function of lipin-1 as a co-transcriptional regulator is seen in the formation of adipose tissue and triacylglycerol storage. Lipin-1 does this by regulating the expression of the transcriptional regulator, PPARγ, which is necessary for the differentiation of pre-adipocytes [8]. The importance of this regulation is highlighted by the absence of mature adipose tissue in the lipin-1 deficient fld mice.
Subcellular Localization of Lipins and Their Regulation
It should be noted that most of research to date has been focused on lipin-1 and, therefore, the following sections will concentrate on this isoform. There are two splice variants of lipin-1, full-length lipin-1b and lipin-1a (which is missing a stretch of 22 amino acids). Both isoforms appear to possess both PAP and transcriptional co-activator functions [1,4]. Therefore, we will use the term “lipin-1” to denote both lipin-1a and -1b. It is interesting to note that the expression of the two splice variants of lipin-1 changes depending on tissue type [8]. There is also a third lipin that is enriched in the brain called lipin-1γ, which appears to be associated with lipid droplets [9]. However, a detailed characterization of the importance of this isoform is lacking. Lipins appear to function as both homo- and hetero-oligomers at the endoplasmic reticulum with each unit in the oligomer having independent PAP activities [10] (Fig. 2). Additionally, the ability to oligomerize can dictate subcellular localization. For example, lipin-1 fused to a CAAX domain can force its recruitment to the plasma membrane; the CAAX domain is post-translationally modified with an isoprenoid moiety, which promotes membrane association [10]. This promotes the association of other lipins to the plasma membrane by oligomerization with the CAAX-tagged lipin-1.
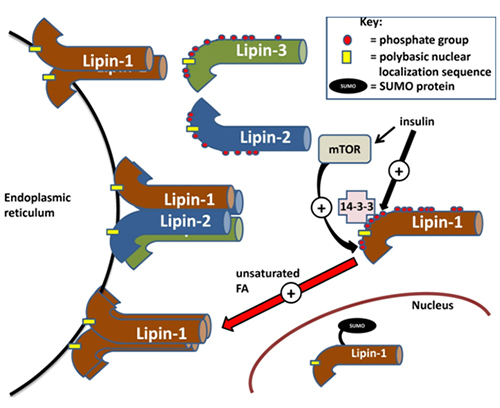
The lipins are the only enzymes of glycerolipid synthesis that are localized to the cytosol; the other proteins in this pathway are all integral membrane proteins (Fig. 1). Consequently, the lipins need to translocate from the cytosol to membranes, particularly the endoplasmic reticulum, which is the major site of glycerolipid synthesis, to access the phosphatidate that is formed [1] (Fig. 2). Translocation of PAP does not appear to be ATP-dependent as shown in an in vitro assay absent of ATP [11]. All three mammalian lipins contain a polybasic stretch of positively charged amino acids, and this has also been called the “nuclear localization sequence” (NLS). This sequence appears to control the association with membranes, including those of the endoplasmic reticulum [12] possibly by interaction with negative charges (Fig. 2). This finding is compatible with earlier work showing that PAP activity translocates to the endoplasmic reticulum in what appeared to be an electrostatic response to an increase in negative charge on the membranes donated by fatty acids, acyl-CoA esters or phosphatidate [13]. Later work showed that the translocation is promoted preferentially by unsaturated fatty acids and that saturated fatty acids have relatively little effect [11] (Fig. 2). This feed-forward signal from fatty acids enables the rate of triacylglycerol synthesis in cells to increase in proportion to the fatty acid load [reviewed in 14]. Conversely, an increase in positive charge on the endoplasmic reticulum membranes through the association of amphiphilic cationic drugs displaces PAP from the membranes [11], leading to an increased accumulation of phosphatidate and a diversion of lipid synthesis towards the production of acidic phospholipids [15]. Moreover, the polybasic NLS sequence governs lipin-1 translocation to the nucleus and presumably this facilitates its transcriptional co-activator function [12]. Another post-translational modification called SUMOylation promotes nuclear localization of lipin-1 and is required for its transcriptional co-activator function [reviewed in 16] (Fig. 2); SUMOylation is a reversible post-translational process by which small ubiquitin-like modifier (SUMO) proteins are covalently modified onto proteins, which can then influence protein localization [17].
Figure 2. Regulation of the subcellular localization of lipins. Lipins are sequestered in a cytosolic reservoir in the hyperphosphorylated state. Phosphorylation is induced by insulin signaling, partially through mTORC1 (mammalian target of rapamycin complex 1). After insulin stimulation, 14-3-3 scaffolding proteins can associate with lipin-1 phosphorylated at a serine-rich region downstream of the nuclear localization sequence (NLS). Unsaturated fatty acids (e.g. oleate) promote the association of dephosphorylated lipins with the endoplasmic reticulum membrane, where they oligomerize in homo- or hetero-tetramers or dimers. Membrane association is facilitated by the nuclear localization sequence on the lipins. Lastly, nuclear localization of lipin-1 is mediated by SUMOylation.
Lipin-1 is phosphorylated on at least 19 serine/threonine residues and the extent of the phosphorylation regulates its association with membranes [2]. Most of these phosphorylation sites appear to be conserved in lipin-2 and -3 [2]. The kinases responsible for phosphorylation of these sites have been putatively described based on the presence of minimum kinase recognition motifs in lipins; however, positive identification has yet to be performed. Insulin signalling has been shown to promote hyperphosphorylation of lipin-1 on various serine/threonine residues following mTOR activation, which results in decreased membrane association [reviewed in 1] (Fig. 2). This again supports the hypothesis that membrane-binding is dependent on electrostatic interactions since the overall negative charge of the phosphate groups would interfere with the association of lipin-1 with the negatively charged surface of the endoplasmic reticulum membrane. Besides diminishing the capability for electrostatic interactions with the membrane, insulin-stimulated phosphorylation might induce protein-protein interactions that could sequester lipin-1 in the cytosol. One example of this is the binding of a serine-rich domain downstream of the “nuclear localization sequence” on lipin-1a to 14-3-3 scaffolding proteins, which sequesters lipin-1a in the cytosol [18] (Fig. 2). This binding is promoted by the phosphorylation of several serine residues in the serine-rich domain.
The rapid changes in the phosphorylation state of lipins and the ability of unsaturated fatty acids to translocate lipins to the endoplasmic reticulum or nucleus provide short-term regulation of the PAP and transcriptional co-activator functions of the lipins. These translocations enable cells to increase their capacity for triacylglycerol synthesis and fatty acid oxidation in proportion to the fatty acid supply. In addition to this rapid level of regulation, the expression of the lipins is also dynamically regulated by changes in their synthesis in response to altered hormonal status.
Transcriptional Regulation of Lipin-1
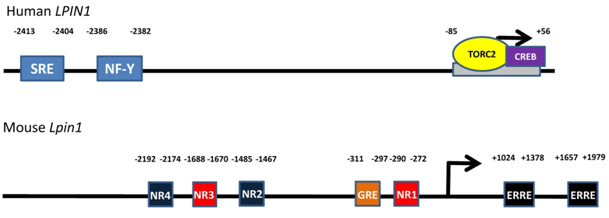
Transcription of the Lpin1 gene in mouse or rat hepatocytes and adipocytes is stimulated by glucocorticoids (steroid hormones produced by the adrenal gland in response to stress and starvation). Activated glucocorticoid receptors translocate into the nucleus and bind to the glucocorticoid receptor element upstream of the mouse Lpin1 promoter [reviewed in 1] (Fig. 3). Stress and starvation are accompanied by increases in glucagon and adrenaline, respectively. Significantly, the glucocorticoid-induced increase in lipin-1 expression is synergized by cyclic AMP (the second messenger generated by glucagon and adrenaline through β-adrenergic receptor signaling). The exact mechanisms underlying this synergism are still unclear. However, it is interesting to note that glucagon or CPT-cAMP (a nondegradable and cell-permeable cAMP analogue) by themselves do not increase Lpin1 expression significantly in hepatocytes. However, in direct contrast, β2-adrenergic receptor stimulation by formoterol in both skeletal muscle in vivo and in cultured myoblasts can increase Lpin1 transcription by causing the activation of nuclear orphan receptor-1, downstream of protein kinase A and CREB signaling (cAMP response element binding protein) [reviewed in 1] (Fig. 3). This discrepancy suggests that transcriptional regulation of Lpin1 downstream of β-adrenergic signaling is receptor specific.
Figure 3. Transcriptional regulatory sites on the LPIN1 (human)/Lpin1 (mouse) gene. Sterol regulatory element binding protein-1 (SREBP1) and nuclear factor-Y bind to the sterol response element (SRE) and nuclear factor-Y (NF-Y) binding site on the LPIN1 gene, respectively. Transducer of regulated CREB activity 2 (TORC2) and cAMP response element binding protein (CREB) bind to the -85 to +56 region on LPIN1. Nuclear orphan receptor-1 binds to nuclear orphan receptor half-sites 1 and 3 (NR1 and 3) on Lpin1 as well as NR2 and 4 on the reverse strand. Glucocorticoid receptors bind to the glucocorticoid response element (GRE) upstream of the Lpin1 promoter and estrogen-related receptors α and γ bind to the estrogen-related receptor elements downstream of the promoter.
The synergistic upregulation of Lpin1 expression by glucocorticoids and cAMP is antagonized by insulin action [1,19]. The mechanism of insulin antagonism has not yet been established. It should also be noted that glucocorticoid-induced Lpin1 expression is partially attenuated if PGC-1α is completely absent [20]. Significantly, the combination of glucocorticoids with cAMP synergistically increased mRNA expression for PGC-1α in rat and mouse hepatocytes and glucocorticoids increased mRNA expression of PPARα [19]. These effects occurred at the same time as the increases in mRNA expression for lipin-1. Also, insulin did not antagonize the increases in mRNA for PGC-1α and PPARα. Furthermore, a recent study has shown that regulation of Lpin1 expression through PGC-1α is mediated by estrogen-related receptors α and γ (ERRα and γ) [21] (Fig. 3).
Activated TORC2 (transducer of regulated CREB activity 2) can increase Lpin1 gene expression in combination with CREB [22] (Fig. 3). TORC2 is dephosphorylated at Ser 171 to induce entry into the nucleus. Dephosphorylation is stimulated by glucagon and fasting whereas phosphorylation is induced by insulin and feeding [22]. It is clear that TORC2 could partially mediate the transcriptional response of the Lpin1 gene to regulation by cAMP- and insulin-dependent signaling. More studies will have to be performed in order to establish a clearer picture of all the interactions involved.
Transcriptional regulation of the gene encoding lipin-1 can also occur through sterol response element binding protein-1 (SREBP-1) [23]. LPIN1 expression in human hepatoblastomas is increased by the action of SREBP-1 in combination with its obligatory co-regulator nuclear factor-Y (NF-Y) binding to a sterol response element and NF-Y binding site, respectively (Fig. 3). SREBP-1 is cleaved when cellular sterol levels are low and translocates into the nucleus where it increases the lipogenic gene program.
The Role of Lipins in Whole Body Metabolism and Their Dysregulation in Diabetes
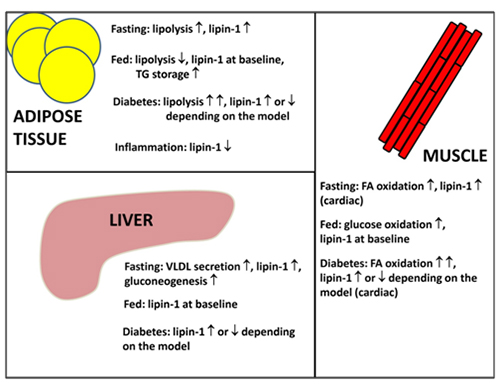
The regulation of lipin-1 by both acute and transcriptional means allows us to understand how lipin-1 integrates into whole body metabolism. In the fasting, stressed and diabetic states, adipose tissue lipolysis is stimulated (Fig. 4) by the action of the catecholamines, epinephrine and norepinephrine (adrenaline and noradrenaline), leading to elevated plasma fatty acid levels. These changes enable the liver, and skeletal and cardiac muscle to increase their use of fatty acids as a fuel and spare the use of glucose (Fig. 4). This diverts glucose to the brain, which is an obligate user of glucose and ketones (from fatty acid oxidation in starvation). The liver also increases glucose secretion in starvation by increasing the synthesis of key gluconeogenic enzymes and enzymes involved in amino acid catabolism. Significantly, the increase in the expression of these enzymes (e.g., pyruvate carboxylase, phosphoenolpyruvate carboxykinase and tyrosine amino transferase) parallels the regulation of lipin-1 [reviewed in 1]. Expression is increased by glucocorticoids and glucagon (through cAMP) and decreased insulin signalling. The increased expression of lipin-1 together with the fatty acid-induced translocation of lipin-1 and -2 to the endoplasmic reticulum facilitate increased TG synthesis in starvation, diabetes and stress conditions. This enables fatty acids that arrive at the liver in excess of the requirements for β-oxidation to be stored temporarily as fat droplets resulting in a fatty liver. Increased expression of lipin-1 should also increase the capacity for β-oxidation through its function as a co-transcriptional regulator and inducer of the expression of proteins required for fatty acid uptake and oxidation. This latter process involves the interaction of lipin-1 with PGC-1α and PPARα, both of which are also induced by fasting [20,24]. Additionally, the absence of insulin signaling would also allow increased lipin-1 nuclear localization [18]. In fact, there is evidence that TG synthesis followed by lipolysis are necessary processes that are required to sustain optimal levels of fatty acid oxidation [reviewed in 14]. Consequently, triacylglycerol synthesis should be viewed as companion pathways to β-oxidation rather than antagonistic events.
Figure 4. Overview of lipins in the control of whole-body metabolism. During fasting, lipolysis in adipose tissue increases and fatty acid utilization by the liver, heart and skeletal muscle increases. Furthermore, very-low-density lipoprotein (VLDL) secretion from liver and the gluconeogenic program also increase. Lipin-1 expression in adipose tissue, muscle and liver is upregulated during fasting to accommodate increased fatty acid flux and triacylglycerol cycling. In the fed state, lipin-1 expression returns to baseline, adipose tissue lipolysis decreases and TG storage increases. Fatty acid utilization in liver and muscle decrease at the expense of glucose oxidation. In the diabetic state, insulin signaling is low and the conditions resemble an exacerbated fasting state. Lipin-1 expression has been shown to be increased or decreased depending on the disease model. One compensatory mechanism in a diabetic state with increased fatty acid flux is probably through increasing lipin-1 expression to increase the capacity for fatty acid storage as well as utilization. Conversely, the occurrence of decreased lipin-1 expression is probably due to chronic metabolic dysregulation and maladaptation, as well as increased inflammatory signaling.
The TG that is stored temporarily in the liver in cytosolic droplets is also hydrolyzed to provide fatty acids that are then re-esterified to TG for secretion in very-low-density lipoproteins (VLDL) (Fig. 4). Significantly, glucocorticoids also increase the rate of VLDL secretion from hepatocytes and this effect is antagonized by insulin [25]. One component of this regulation is mediated through the increased expression of lipin-1 [26], and additionally, glucocorticoids also increase the stability and secretion of apolipoprotein B [27]. The role of insulin as a regulator of VLDL secretion has been controversial and there is now a general consensus that insulin, at least in the short term, inhibits this process, thus contributing to the association of insulin resistance with hypertriglyceridemia. Although increased lipin-1 expression is involved in stimulating VLDL secretion, this process is high in livers of fld mice, which are lipin-1 deficient [reviewed in 1]. These mice exhibit hypertriglyceridemia during the weaning period and, therefore, other lipins, probably lipin-2, are also responsible for facilitating VLDL secretion.
During feeding, glucagon levels are decreased and insulin production is stimulated. Dietary fatty acids are taken up in the intestine and packaged into chylomicrons, which are then secreted into the circulatory lymphatic system. Uptake and oxidation of glucose increase and adipose tissue lipogenesis is promoted (Fig. 4). The roles of lipins in chylomicron packaging and adipose tissue lipogenesis are not clear; however, it is quite possible that lipin-1 and -3 could play important roles given that they are most highly expressed in adipose tissue and intestines, respectively. Lipin-1 expression in liver and muscle returns to baseline due to increased insulin signaling, which would facilitate the switch from fatty acid to glucose utilization (Fig. 4). It should be noted that overexpression of lipin-1 in skeletal muscle leads to insulin-resistant, obese mice, pointing to the importance of lipin-1 in regulating muscle energy homeostasis [reviewed in 1].
Metabolic dysregulation that occurs in disease states would perturb the homeostatic control of substrate utilization, e.g. the onset of type 2 diabetes. In the diabetic condition, insulin signaling is severely impaired leading to excessive adipose tissue lipolysis and gluconeogenesis [reviewed in 14]. Glucose utilization in muscle is reduced and fatty acid oxidation is highly upregulated [reviewed in 14]. Under these conditions, lipin-1 expression is highly induced [reviewed in 14]. All the changes occurring in type 2 diabetes are thought to be compensatory responses; however, chronic metabolic imbalance leads to pathological outcomes. As an example, a build-up in inflammatory responses leads to the downregulation of lipin-1 expression in adipocytes [reviewed in 16](Figure 4). Additionally, decreased lipin-1 expression has been shown in tissue biopsies from the livers and adipose tissue of some human patients and this is inversely correlated to insulin sensitivity [reviewed in 1].
Mutations and Polymorphisms in LPIN Genes and Their Association to Various Diseases
The first model demonstrating a spontaneously occurring mutation in the Lpin1 gene was the fatty liver dystrophy or fld mouse [reviewed in 1]. This mouse has a transient fatty liver and is hypertriglyceridemic, both of which resolve upon weaning [1]. Fld mice also lack adipose tissue (lipodystrophic), insulin-resistant and susceptible to diet-induced atherosclerosis [1]. Finally, the mice undergo progressive peripheral neuropathy due to demyelination in the Schwann cells because of phosphatidate accumulation and ERK activation [reviewed in 1]. These results and other work suggests that lipins could be involved in regulating signal transduction as well as glycerolipid synthesis since phosphatidate is known to control several signalling targets [reviewed in 28]. Deficiency of lipin-1 in human beings produces a different phenotype where a subset of patients is prone to early-onset breakdown of muscle fibers (rhabdomyolysis) leading to the release of myoglobin into the blood and urine (myoglobinemia) because of homozygous recessive or heterozygous mutations in the LPIN1 gene [29]. Unlike the majority of patients with this condition, patients with LPIN1 gene mutations do not have any mitochondrial defects or reductions in the capacity for FA oxidation.
Homozygous recessive mutations in LPIN2 have also been linked to a genetic disease called the Majeed syndrome [reviewed in 1]. Patients suffering from Majeed syndrome are characterized by recurrent fevers and inflammation in the bones and skin, and a form of anemia known as congenital dyserythropoietic anemia [1]. The Majeed mutation of lipin-2 results in a catalytically inactive PAP in the absence of a defect in its co-transcriptional regulatory capacity [3]. So far, no known disease state has been associated with the LPIN3 gene.
In addition to genetic mutations in the LPIN genes, several studies have associated gene polymorphisms with metabolic phenotypes. LPIN1 gene single nucleotide polymorphisms have been negatively associated to serum insulin levels, circulating cholesterol in low-density lipoproteins [reviewed in 1], and the occurrence of diabetes and obesity [reviewed in 1]. Similar associations to insulin sensitivity and the occurrence of type 2 diabetes have been found with LPIN2 single nucleotide polymorphisms [reviewed in 1]. Conversely, there are other loci containing LPIN1 single nucleotide polymorphisms that positively correlate to lower blood pressure, decreased waist circumference, and a reduced occurrence of the metabolic syndrome [reviewed in 1]. However, it should also be noted that there have been studies demonstrating little or no association of LPIN1 gene polymorphisms to deleterious metabolic phenotypes [30]. Studies on LPIN3 have not been performed.
Nonmammalian Lipins – Regulation, Function and Applicability to Humans
Tremendous insight regarding the function and regulation of lipins in mammals can be gained by examining the research conducted on nonmammalian systems. For instance, the discovery that mammalian lipin-1 possesses PAP activity only occurred due to the characterization of lipin in Saccharomyces cerevisiae as a PAP enzyme [31]. The nonmammalian systems examined, e.g. S. cerevisiae and Arabidopsis thaliana,possess either 1 or 2 lipin isoforms [reviewed in 16]. The physiological attributes of these organisms have been extensively researched and are well defined, which provides a significant advantage in determining the cellular role of lipins. However, it is also acknowledged that results obtained in lower organisms do not completely translate to more complicated mammalian systems. For example, the catalytic activity of S. cerevisiae lipin regulates its cellular roles in transcriptional regulation [32], which is not true for mammals.
Studies in S. cerevisiae and Caenorhabditis elegans demonstrate the essential requirement of lipin in maintaining endoplasmic reticulum and nuclear membrane homeostasis [reviewed in 16]. Like its mammalian homologue, the phosphorylation state, cell localization and PAP activity of S. cerevisiae lipin control its function [reviewed in 16,32]. Phosphorylation and dephosphorylation of S. cerevisiae lipin is catalyzed by cyclin-dependent Cdc28p kinase [reviewed in 16] and the Nem1p-Spo7p phosphatase [reviewed in 1], respectively. The human homologue of the yeast phosphatase, i.e. Dullard has also been shown to dephosphorylate lipin-1 [reviewed in 1], although the physiological consequences of lipin regulation by Dullard in mammals have yet to be elucidated. Moreover, both lipin-1 and -2 have been shown to be phosphorylated, and regulated by cyclin-dependent kinase 1 during mitosis, possibly as a means of regulating phospholipid production [33].
Conclusions
Lipins are unique bifunctional proteins involved in the control of both glycerolipid synthesis and fatty acid oxidation through their phosphatidate phosphatase and transcriptional co-activator activities. Mammalian lipins are essential proteins that have been shown to produce disease phenotypes if they are mutated or dysfunctional. Elucidation of the regulation of lipins and the roles of the different lipins will greatly improve our understanding of the regulation of fatty acid and glycerolipid metabolism.
Bibliography
- Reue, K. and Brindley, D.N. Thematic Review Series: Glycerolipids. Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism. J. Lipid Res., 49, 2493-2503 (2008) (DOI: 10.1194/jlr.R800019-JLR200).
- Harris, T.E., Huffman, T.A., Chi, A., Shabanowitz, J., Hunt, D.F., Kumar, A. and Lawrence, J.C. Insulin controls subcellular localization and multisite phosphorylation of the phosphatidic acid phosphatase, lipin 1. J. Biol. Chem., 282, 277-286 (2007) (DOI: 10.1074/jbc.M609537200).
- Donkor, J., Zhang, P., Wong, S., O'Loughlin, L., Dewald, J., Kok, B.P., Brindley, D.N. and Reue, K. A conserved serine residue is required for the phosphatidate phosphatase activity but not the transcriptional coactivator functions of lipin-1 and lipin-2. J. Biol. Chem., 284, 29968-29978 (2009) (DOI: 10.1074/jbc.M109.023663).
- Donkor, J., Sariahmetoglu, M., Dewald, J., Brindley, D.N. and Reue, K. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J. Biol. Chem., 282, 3450-3457 (2007) (DOI: 10.1074/jbc.M610745200).
- Gropler, M.C., Harris, T.E., Hall, A.M., Wolins, N.E., Gross, R.W., Han, X., Chen, Z. and Finck, B.N. Lipin 2 is a liver-enriched phosphatidate phosphohydrolase enzyme that is dynamically regulated by fasting and obesity in mice. J. Biol. Chem., 284, 6763-6772 (2009) (DOI: 10.1074/jbc.M807882200).
- Lin, J., Handschin, C. and Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab., 1, 361-370 (2005) (DOI: 10.1016/j.cmet.2005.05.004).
- Banke, N.H., Wende, A.R., Leone, T.C., O'Donnell, J.M., Abel, E.D., Kelly, D.P. and Lewandowski, E.D. Preferential oxidation of triacylglyceride-derived fatty acids in heart is augmented by the nuclear receptor PPARα. Circ. Res., 107, 233-241 (2010) (DOI: 10.1161/CIRCRESAHA.110.221713).
- Peterfy, M., Phan, J. and Reue, K. Alternatively spliced lipin isoforms exhibit distinct expression pattern, subcellular localization, and role in adipogenesis. J. Biol. Chem., 280, 32883-32889 (2005) (DOI: 10.1074/jbc.M503885200).
- Wang, H., Zhang, J., Qiu, W., Han, G.S., Carman, G.M. and Adeli, K. Lipin-1γ isoform is a novel lipid droplet-associated protein highly expressed in the brain. FEBS Lett., 585, 1979-1984 (2011) (DOI: 10.1016/j.febslet.2011.05.035).
- Liu, G.H., Qu, J., Carmack, A.E., Kim, H.B., Chen, C., Ren, H., Morris, A.J., Finck, B.N. and Harris, T.E. Lipin proteins form homo- and hetero-oligomers. Biochem. J., 432, 65-76 (2010) (DOI: 10.1042/BJ20100584).
- Hopewell, R., Martin-Sanz, P., Martin, A., Saxton, J. and Brindley, D.N. Regulation of the translocation of phosphatidate phosphohydrolase between the cytosol and the endoplasmic reticulum of rat liver. Effects of unsaturated fatty acids, spermine, nucleotides, albumin and chlorpromazine. Biochem. J., 232, 485-491 (1985).
- Ren, H., Federico, L., Huang, H., Sunkara, M., Drennan, T., Frohman, M.A., Smyth, S.S. and Morris, A.J. A phosphatidic acid binding/nuclear localization motif determines lipin-1 function in lipid metabolism and adipogenesis. Mol. Biol. Cell, 21, 3171-3181 (2010) (DOI: 10.1091/mbc.E10-01-0073).
- Martin-Sanz, P., Hopewell, R. and Brindley, D.N. Long-chain fatty acids and their acyl-CoA esters cause the translocation of phosphatidate phosphohydrolase from the cytosolic to the microsomal fraction of rat liver. FEBS Lett., 175, 284-288 (1984).
- Brindley, D.N., Kok, B.P., Kienesberger, P.C., Lehner, R. and Dyck, J.R. Shedding light on the enigma of myocardial lipotoxicity: the involvement of known and putative regulators of fatty acid storage and mobilization. Am. J. Physiol. Endocrinol. Metab., 298, E897-E908 (2010) (DOI: 10.1152/ajpendo.00509.2009).
- Brindley, D.N., Allan, D. and Michell, R.H. Letter: The redirection of glyceride and phospholipid synthesis by drugs including chlorpromazine, fenfluramine, imipramine, mepyramine and local anaesthetics. J. Pharm. Pharmacol., 27, 462-464 (1975).
- Harris, T.E. and Finck, B.N. Dual function lipin proteins and glycerolipid metabolism. Trends Endocrinol. Metab., 22, 226-233 (2011) (DOI: 10.1016/j.tem.2011.02.006).
- Gareau, J.R. and Lima, C.D. The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat. Rev. Mol. Cell. Biol., 11, 861-871 (2010) (DOI: 10.1038/nrm3011).
- Peterfy, M., Harris, T.E., Fujita, N. and Reue, K. Insulin-stimulated interaction with 14-3-3 promotes cytoplasmic localization of lipin-1 in adipocytes. J. Biol. Chem., 285, 3857-3864 (2010) (DOI: 10.1074/jbc.M109.072488).
- Manmontri, B., Sariahmetoglu, M., Donkor, J., Khalil, M.B., Sundaram, M., Yao, Z., Reue, K., Lehner, R. and Brindley, D.N. Glucocorticoids and cyclic AMP selectively increase hepatic lipin-1 expression, and insulin acts antagonistically. J. Lipid. Res., 49, 1056-1067 (2008) (DOI: 10.1194/jlr.M800013-JLR200).
- Finck, B.N., Gropler, M.C., Chen, Z., Leone, T.C., Croce, M.A., Harris, T.E., Lawrence, J.C. and Kelly, D.P. Lipin 1 is an inducible amplifier of the hepatic PGC-1α/PPARα regulatory pathway. Cell Metab., 4, 199-210 (2006) (DOI: 10.1016/j.cmet.2006.08.005).
- Mitra, M.S., Schilling, J.D., Wang, X., Jay, P.Y., Huss, J.M., Su, X. and Finck, B.N. Cardiac lipin 1 expression is regulated by the peroxisome proliferator activated receptor γ coactivator 1α/estrogen related receptor axis. J. Mol. Cell. Cardiol., 51, 120-128 (2011) (DOI: 10.1016/j.yjmcc.2011.04.009).
- Ryu, D., Oh, K.J., Jo, H.Y., Hedrick, S., Kim, Y.N., Hwang, Y.J., Park, T.S., Han, J.S., Choi, C.S., Montminy, M. and Koo, S.H. TORC2 regulates hepatic insulin signaling via a mammalian phosphatidic acid phosphatase, LIPIN1. Cell Metab., 9, 240-251 (2009) (DOI: 10.1016/j.cmet.2009.01.007).
- Ishimoto, K., Nakamura, H., Tachibana, K., Yamasaki, D., Ota, A., Hirano, K., Tanaka, T., Hamakubo, T., Sakai, J., Kodama, T. and Doi, T. Sterol-mediated regulation of human lipin 1 gene expression in hepatoblastoma cells. J. Biol. Chem., 284, 22195-22205 (2009) (DOI: 10.1074/jbc.M109.028753).
- Finck, B.N. and Kelly, D.P. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J. Clin. Invest., 116, 615-622 (2006) (DOI: 10.1172/JCI27794).
- Martin-Sanz, P., Vance, J.E. and Brindley, D.N. Stimulation of apolipoprotein secretion in very-low-density and high-density lipoproteins from cultured rat hepatocytes by dexamethasone. Biochem. J., 271, 575-583 (1990).
- Bou Khalil, M., Blais, A., Figeys, D. and Yao, Z. Lipin - The bridge between hepatic glycerolipid biosynthesis and lipoprotein metabolism. Biochim. Biophys. Acta, 1801, 1249-1259 (2010) (DOI: 10.1016/j.bbalip.2010.07.008).
- Wang, C.N., McLeod, R.S., Yao, Z. and Brindley, D.N. Effects of dexamethasone on the synthesis, degradation, and secretion of apolipoprotein B in cultured rat hepatocytes. Arterioscler. Thromb. Vasc. Biol., 15, 1481-1491 (1995).
- Brindley, D.N., Pilquil, C., Sariahmetoglu, M. and Reue, K. Phosphatidate degradation: Phosphatidate phosphatases (lipins) and lipid phosphate phosphatases. Biochim. Biophys. Acta, 1791, 956-961 (2009) (DOI: 10.1016/j.bbalip.2009.02.007).
- Zeharia, A., Shaag, A., Houtkooper, R.H., Hindi, T., de Lonlay, P., Erez, G., Hubert, L., Saada, A., de Keyzer, Y., Eshel, G., Vaz, F.M., Pines, O. and Elpeleg, O. Mutations in LPIN1 cause recurrent acute myoglobinuria in childhood. Am. J. Hum. Genet., 83, 489-494 (2008) (DOI: 10.1016/j.ajhg.2008.09.002).
- Burgdorf, K.S., Sandholt, C.H., Sparso, T., Andersen, G., Witte, D.R., Jorgensen, T., Sandbaek, A., Lauritzen, T., Sorensen, T.I., Madsbad, S., Hansen, T. and Pedersen, O. Studies of association between LPIN1 variants and common metabolic phenotypes among 17,538 Danes. Eur. J. Endocrinol., 163, 81-87 (2010) (DOI: 10.1530/EJE-10-0068).
- Han, G.S., Wu, W.I. and Carman, G.M. The Saccharomyces cerevisiae lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J. Biol. Chem., 281, 9210-9218 (2006) (DOI: 10.1074/jbc.M600425200).
- Han, G.S., Siniossoglou, S. and Carman, G.M. The cellular functions of the yeast lipin homolog PAH1p are dependent on its phosphatidate phosphatase activity. J. Biol. Chem., 282, 37026-37035 (2007) (DOI: 10.1074/jbc.M705777200).
- Grimsey, N., Han, G.S., O'Hara, L., Rochford, J.J., Carman, G.M. and Siniossoglou, S. Temporal and spatial regulation of the phosphatidate phosphatases lipin 1 and 2. J. Biol. Chem., 283, 29166-29174 (2008) (DOI: 10.1074/jbc.M804278200).
In This Section
- Glycerophosphate and Acylglycerophosphate Acyltransferases
- Mammalian Diacylglycerol Acyltransferases (DGAT)
- Fatty Acid beta-Oxidation
- Regulation of Lipins and Their Role in Lipid Metabolism
- Phospholipid Biosynthesis
- Phospholipases
- Acylglycerol Lipases (Neutral Lipid Hydrolysis)
- Metabolism and Function of Very-Long-Chain Polyunsaturated Fatty Acids (>C24) in Mammals