Acylglycerol Lipases (Neutral Lipid Hydrolysis)
The Authors: Ariel D. Quiroga and Richard Lehner DOI: 10.21748/lipidlibrary.39188
Introduction
Triacylglycerol (triglyceride, TG) hydrolysis represents a crucial step in the absorption and redistribution of energy in mammals. TG contains three fatty acids esterified to a glycerol backbone. Fatty acids are a rich source of energy and their oxidation yields twice as much energy per gram compared to glucose. Fatty acids for the synthesis of TG are derived from the diet, from de novo synthesis, or from other endogenous lipids. The glycerol backbone is supplied either from glucose via glycolysis or by gluconeogenesis, and it enters the synthetic TG pathway in the form of glycerol-3-phosphate.
Alternatively, the backbone can be derived from incomplete hydrolysis of dietary TG and enters the synthetic pathway in the form of 2-monoacylglycerol (MG). Dietary TG is hydrolyzed in the stomach and the small intestine by digestive lipases. This process is necessary for efficient absorption of fat and fat-soluble vitamins. Pharmacological inhibition of digestive lipases by orlistat (tetrahydrolipstatin, Xenical) leads to malabsorption and weight loss and is used to treat obesity. Absorbed fatty acids and 2-MG recombine in the intestinal cells through the action of enzymes called MG and diacylglycerol (DG) acyltransferases (MGAT and DGAT) back to TG. This TG is packaged into lipoprotein particles termed chylomicrons, which are in turn exported into the blood. There, lipoprotein lipases again hydrolyze TG within these particles and the fatty acids are delivered to various tissues mainly for storage (adipose tissue) but also for energy utilization (muscle, heart, liver, etc.). Lipoprotein-derived fatty acids that have entered the cells are again re-esterified to TG.
When energy is required, TG stored in the lipid droplets within cells is hydrolyzed, and fatty acids are oxidized in the mitochondria to produce ATP. Excessive TG storage in adipocytes leads to obesity and increased fatty acid release from the adipose tissue storage pools. This results in increased delivery of fatty acids to organs including the liver, muscle and the heart. These organs are not equipped to store large amounts of fatty acids, and pathologies such as fatty liver (nonalcoholic fatty liver disease, nonalcoholic steatohepatitis), insulin resistance, type 2 diabetes and cardiovascular complications can ensue. Therefore, it is important to understand the enzymes regulating TG metabolism both from the physiological and pharmacological point view. This chapter reviews the current knowledge on lipases involved in the hydrolysis of neutral acylglycerol lipids (TG, DG and MG).
General Structural Features of Lipases and Interfacial Activation Kinetics
Although lipases vary greatly in their size and amino acid composition, they are all serine esterases belonging to the α/β hydrolase superfamily. The α/β hydrolase classification alludes to the secondary structure of the proteins, where α-helices follow the β-sheets with the central β-sheet core containing at least five parallel strands. In the majority of the lipases the nucleophilic serine residue is part of a Ser-Asp/Glu-His triad (in this order in the primary amino acid sequence), but some lipase active sites contain a Ser-Asp dyad. The catalytic Ser residues lie within the GXSXGA/G lipase/esterase signature motif. Another structural feature of many lipases is the presence of a loop (“lid”) domain that protects the hydrophobic entry to the active site from the aqueous environment. The lid also plays an important role in substrate selectivity.
Lipases are soluble enzymes, yet they act on insoluble substrates at the water/lipid interface and follow interfacial activation kinetics rather than the canonical Michaelis-Menten kinetics. The catalytic mechanism depends strongly on the organization of lipids at the interface, as well as lipid composition that may affect the physical aspect of the surface at which the lipases act: membrane bilayers (organelles), monolayers (lipid dropletss, lipoproteins), micelles (intestinal absorption) or oil-in water emulsions. The adsorption of lipases to lipid surfaces necessitates significant structural rearrangement of the proteins. The opening of the lid domain creates a large hydrophobic surface and provides an entry for the substrate to the active site. The “open lid” conformation of lipases at the lipid interface is stabilized through an interaction of the lipase with a co-factor (sometimes called a colipase or an activating protein), which enhances the catalytic activity of the lipase.
Digestive Triacylglycerol Lipases (Fat Absorption)
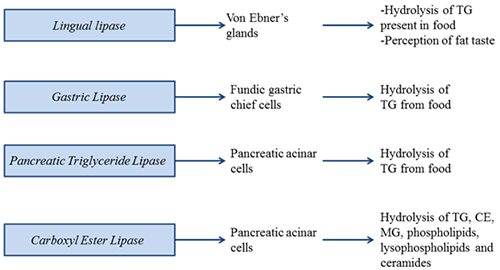
Lingual lipase is secreted from von Ebner’s glands. It initiates the lipolysis of fat in the oral cavity. Lingual lipase shares protein sequence identity with gastric lipase, released from gastric mucosa. Lingual lipase is present in rat, mice, human and cattle. However, very little activity was found in humans, where other lipase activities predominate. Therefore, lingual lipase is thought to be less important for fat digestion and absorption in healthy adult mammals, and a role in perception of fat taste has been addressed.
Figure 1. Digestive triacylglycerol lipases involved in fat absorption and signaling.
Gastric lipase initiates the lipolysis of dietary fat in the stomach. Gastric lipase is a unique enzyme because it is fully active in the gastric juice, which has a pH of about 2. In order to be stable at this low pH, this lipase is highly glycosylated with glycan moiety accounting for about 15% of the mass of the protein. The lipase belongs to the family that also includes lysosomal acid lipase, which will be discussed later. Gastric lipase does not require an activating co-factor for its catalytic activity and preferentially hydrolyzes the sn-3-position in TG, yielding sn-1,2-DG as the product. The overall contribution to fat digestion is estimated to be about 20%. Because of its stability, and its co-factor independence for catalysis, gastric lipase was chosen as an enzyme replacement therapy to treat pancreatic insufficiency observed in chronic pancreatitis and cystic fibrosis.
Pancreatic triglyceride lipase is secreted by the pancreatic acinar cells into the duodenum, where it digests dietary lipids (TG and DG) into 2-MG and fatty acids. Three subgroups of human pancreatic triglyceride lipase have been identified (pancreatic triglyceride lipase, pancreatic triglyceride lipase related protein-1 and pancreatic triglyceride lipase related protein-2) sharing about 70% sequence identity. The main TG lipase in adults is pancreatic triglyceride lipase. Pancreatic triglyceride lipase related protein-1 does not exhibit a TG lipase activity, while pancreatic triglyceride lipase related protein-2 is responsible for the absorption of dietary fat in suckling mice. Pancreatic triglyceride lipase requires a colipase for anchoring to lipid-containing micelles and for protection against inactivation by bile salts. This 11 kDa protein is secreted from pancreatic acinar cells as procolipase, and is processed by proteolytic cleavage to colipase and a pentapeptide called enterostatin that has been suggested to play a role in appetite control in animals. Ablation of colipase gene expression in mice results in decreased postnatal survival, indicating the importance of this co-factor in fat absorption.
Pancreatic triglyceride lipase secretion from pancreatic acinar cells is stimulated by secretagogues including cholecystokinin and acetylcholine. Cholecystokinin also appears to be the major signal regulating bile salt secretion from the gallbladder. Bile acid-mediated emulsification and stabilization of lipid-containing micelles is important for efficient pancreatic triglyceride lipase function. Interestingly, despite the undisputed importance of pancreatic triglyceride lipase in fat digestion, its genetic ablation in mice did not result in fat malabsorption even when mice were fed high-fat diet. However, postprandial fat absorption was only delayed, not decreased, in pancreatic triglyceride lipase deficiency and the animals adapted by absorbing fat across the entire length of the small intestine, rather than just the proximal jejunum. The lack of malabsorption in pancreatic triglyceride lipase-deficient animals can be explained by compensatory mechanisms involving pancreatic triglyceride lipase related protein-2 and carboxyl ester lipase.
Carboxyl ester lipase, also termed pancreatic cholesterol esterase, is a bile salt-stimulated (or –activated or –dependent) lipase that hydrolyzes not only TG but also cholesteryl esters (CE), MG, phospholipids, lysophospholipids and ceramides. Like pancreatic triglyceride lipase, carboxyl ester lipase is also secreted by the pancreas after a meal. Genetic ablation of carboxyl ester lipase expression in mice does not affect dietary TG absorption but decreases the production and size of chylomicron particles. Carboxyl ester lipase deficiency results in 60% decrease of CE absorption but has no direct effect on the absorption of unesterified cholesterol. Low amounts of carboxyl ester lipase have also been shown to be secreted into the circulation by the macrophages; however, the physiological significance of circulating carboxyl ester lipase is not clear and awaits experiments using macrophage-specific deletion of carboxyl ester lipase.
Plasma Triacylglycerol Lipases (Lipoprotein Metabolism)
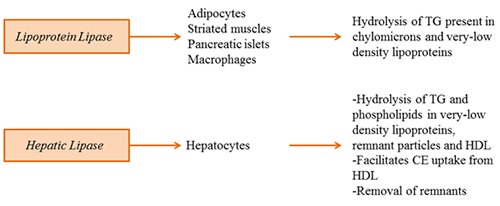
Lipoprotein lipase hydrolyzes TG present in chylomicrons and very-low-density lipoproteins. Loss-of-function mutations result in chylomicronemia (milky plasma). Lipoprotein lipase belongs to the pancreatic triglyceride lipase family of lipases. It is synthesized in adipocytes, cardiac and skeletal muscle, islets and macrophages and is translocated to the luminal surface of endothelial cells, where it docks onto heparin-sulfate proteoglycans and glycosylphosphatidylinositol-anchored high density lipoprotein binding protein 1 (GPIHBP1). GPIHBP1 is crucial not only for the translocation of lipoprotein lipase across the endothelial cell but also for protection of lipoprotein lipase against inhibition by angiopoetin-like protein 4. The catalytically active lipoprotein lipase exists as a head-to-head homodimer. The folding of lipoprotein lipase into its functional form requires the activity of an endoplasmic reticulum-localized chaperone called lipase maturation factor 1. In plasma, lipoprotein lipase is activated by apolipoprotein CII, while angiopoetin-like proteins 3 and 4 and apolipoprotein CIII have been shown to inhibit lipoprotein lipase activity.
Figure 2. Plasma triacylglycerol lipases regulating lipoprotein metabolism.
Lipoprotein lipase is nutritionally regulated in a tissue-specific manner. Its activity is high in adipose tissue and low in the heart and skeletal muscle in the fed state. During feeding, muscles use glucose as the primary source of energy and do not have a need for fatty acid. Fatty acids are therefore deposited for storage in the adipose tissue. During fasting, lipoprotein lipase activity in the heart and the muscle increases, while in the adipose tissue decreases – a signal for utilization of fatty acid as the energy source. While insulin plays a significant role in the regulation of lipoprotein lipase expression/activity in the adipose tissue, the regulation of its expression/activity in the heart appears to be more complex and may be governed by inactivation/dimer dissociation mechanism rather than at the level of gene expression.
Hepatic lipase is mainly expressed in hepatocytes and binds to heparin sulfate proteoglycans on the cell surface of parenchymal cells. Hepatic lipase hydrolyzes both TG and phospholipids in very-low-density lipoprotein, remnant lipoproteins and high-density lipoprotein. Aside from its lipolytic function, hepatic lipase also facilitates selective uptake of CE from high-density lipoproteins as well as removal of lipoprotein remnants. Therefore, hepatic lipase functions in the clearance of lipoproteins from the circulation, yet whether it is pro- or anti-atherogenic remains controversial. This is because high hepatic lipase activity is correlated with the production of small dense atherogenic low-density lipoproteins. On the other hand, it reduces the concentration of circulating TG and apolipoprotein B, two key factors associated with atherogenesis.
Like lipoprotein lipase, hepatic lipase functions as a head-to-tail homodimer and requires lipase maturation factor 1 for proper folding. There is some controversy as to which point along the secretory route hepatic lipase attains its lipolytic activity, but hepatic lipase activity was demonstrated in the intracellular compartment, suggesting that the lipase may also play a role in intracellular lipid metabolism.
Intracellular Triacylglycerol Lipases (Energy Provision and Signaling)
Intracellular lipolysis is complex and compartmentalized into three major locations: cytosol, endoplasmic reticulum and late endosomes/lysosomes. As expected, the origins of the substrates differ among the locations and to some extent also the fate of the lipolytic products. Much has been learned about intracellular lipolysis from studies in the adipocytes. However, care should be taken not to generalize the mechanism of adipose tissue lipolysis to other tissues because different sets of lipases may be employed. Nevertheless, it is important to note that major steps have been taken in the last decade to understand intracellular lipolysis, especially in the adipose tissue, heart and skeletal muscle, in the liver and to some extent also in the small intestine.
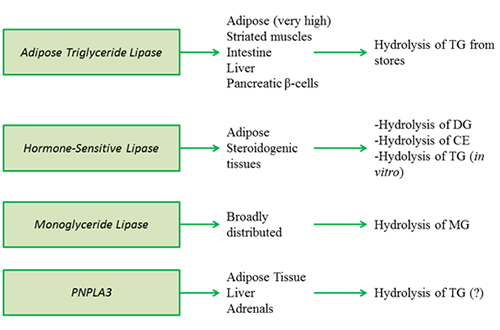
Lipolysis of stored TG in the adipose tissue is regulated by nutritional needs. Lipolysis is suppressed during postprandial state (insulin action predominates), while during fasting (catecholamines and stress hormones predominate) lipolysis of stored TG in the adipose tissue is stimulated. The degradation of TG to fatty acids and glycerol is catalyzed by a sequential action of three lipase:, adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL) and monoglyceride lipase (MGL). A similar sequence of events appears to occur in the heart, muscle and pancreatic β-cells. However, not all tissues cells express ATGL and/or HSL and some tissues/cells express specific lipolytic enzymes.
Figure 3A. Intracellular triacylglycerol lipases involved in energy provision and signaling.
Adipose triglyceride lipase (ATGL) (annotated as patatin-like phospholipase domain containing protein 2, PNPLA2) catalyzes the conversion of TG to DG. The stereospecificity of ATGL for the sn-1, sn-2 or sn-3 positions is not yet entirely clear. ATGL is highly expressed in adipose tissue but also to a lesser extent in other tissues including the heart, muscle, intestine, liver and pancreatic β-cells, suggesting a wider role of this enzyme in energy homeostasis. The enzyme contains a catalytic dyad Ser-Asp. The lipase is active on cytosolic lipid droplets.
The crucial role of ATGL in TG lipolysis in various tissues has been demonstrated in mice in which the gene encoding ATGL has been deleted. ATGL-deficient mice present with increased TG stores in most tissues, but the largest deposits are found in the heart. This exaggerated steatosis leads to impaired heart function and premature death of the animals from cardiac malfunction. The mice also do not respond well to cold stress, implicating the importance of ATGL in provision of fatty acids for thermogenesis and for providing a ligand for transcription factors and transcriptional coactivators regulating fatty acid oxidation (PGC1α and PPARα). ATGL deficiency results in very low release of fatty acids from adipose tissue stores during fasting, which translates to decreased provision of substrates for hepatic very-low-density lipoprotein assembly and low plasma lipid levels. ATGL-deficient animals therefore depend on glucose to satisfy their energy needs and are highly insulin sensitive despite steatosis. Deletion of ATGL only in the liver resulted in 4-fold increase of TG storage without affecting plasma glucose, glucose tolerance, insulin sensitivity or lipid levels, while hepatic fatty acid oxidation was decreased.
ATGL is a hormone-regulated enzyme. It is activated by a co-factor, CGI-58 (annotated as ABHD5). In most tissues, CGI-58 in basal condition associates with perilipin-1 and is not available for activation of ATGL. Lipolytic stimulus results in the production of cAMP, leading to activation of protein kinase A and phosphorylation of perilipin-1. CGI-58 is then released from perilipin-1 and activates ATGL. It is not yet clear which lipid-droplet-associated protein takes the role of perilipin-1 in tissues where perilipin is not expressed (i.e. the liver). CGI-58 plays other roles in addition to ATGL activation, and moonlights as an acylglycerol-3-phosphate acyltransferase.
Hormone-sensitive lipase is the most-studied intracellular lipase. It was believed for a long time that HSL was the only enzyme regulating TG hydrolysis in adipose tissue. However, it has become apparent with the discovery and characterization of ATGL during the past decade that HSL is predominantly a DG lipase (prefers to hydrolyze the sn-3 position to the sn-1 position) and CE lipase, although it can also hydrolyze TG. Despite the demonstrated TG lipase activity in vitro, HSL activity cannot compensate for ATGL deficiency. HSL is mainly expressed in the adipose tissue (both white and brown) and steroidogenic tissues, and to a much lesser extent in other tissues. It is believed to be absent from the human liver, though a low level of expression in mouse livers has been detected.
As with ATGL, HSL activity is increased during fasting. In the basal state HSL is predominantly cytosolic but becomes phosphorylated by protein kinase A during β-adrenergic stimulation, and this posttranslational modification results in its translocation to lipid droplets and activation. The recruitment of HSL to the lipid droplets is dependent on perilipin-1. Mice lacking HSL are not overweight or obese, fatty acid release from adipose tissue is decreased by less than 40% and adipose tissue accumulates DG.
Monoglyceride lipase catalyzes the release of fatty acid from MG and does not exhibit hydrolytic activity towards TG or DG. It is localized in the cytosol and on lipid droplets, but the mechanism that regulates the distribution between the two cellular compartments is currently unknown. Global MGL deficiency in mice resulted in decreased release of fatty acids and glycerol release from white adipose tissue, and consequently diminished hepatic TG levels and very-low-density lipoprotein production.
PNPLA3, also known as adiponutrin and Ca2+-independent phospholipase A2 epsilon (iPLA2ε), shares homology with ATGL (PNPLA2) including the Ser-Asp catalytic dyad, rather than the canonical Ser-Asp/Glu-His triad present in other lipases and esterases. It is expressed in the adipose tissue, liver and adrenal. Like ATGL, HSL and MGL, PNPLA3 is found on lipid droplets and also in other cytoplasmic compartments. PNPLA3 is regulated by nutritional, hormonal and pharmacological factors, but in the opposite direction to ATGL. The concrete physiological substrate of PNPLA3 is not known, however, it was recently shown that PNPLA3 catalyzes the hydrolysis of TG in vitro; therefore, it might also function as a lipase in vivo. Interestingly, little effect on hepatic TG concentrations was observed when the wild-type PNPL3 protein was overexpressed; however, the expression of a mutated PNPLA3 (I148M) variant associated with steatosis promoted hepatic TG accumulation. PNPLA3 ablation (loss of function) did not affect overall body composition, energy homeostasis, hepatic lipid metabolism, glucose homeostasis or insulin sensitivity.
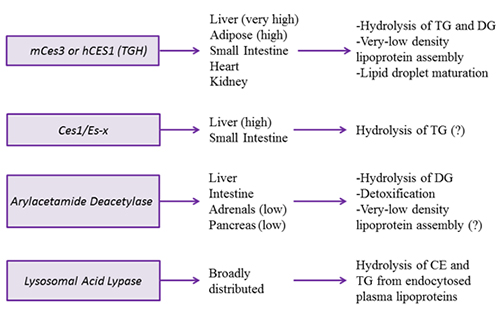
Carboxylesterases (Ces for murine origin or CES for human origin) are localized to the lumen of the endoplasmic reticulum by their C-terminal tetrapeptide sequence HXEL. Carboxylesterase-mediated lipolysis of MG and DG in vitro was already demonstrated about 30 years ago, but the extent of their contribution to lipid hydrolysis in intact cells has not been determined until very recently. Carboxylesterases contain Ser-Glu-His catalytic triad and other hallmarks of lipolytic enzymes, including a hydrophobic crevice lining the entry into the active site and a lid domain.
Figure 3B. Intracellular triacylglycerol lipases involved in energy provision and signaling.
Mouse carboxylesterase Ces3 and its human orthologue CES1, also referred to as triacylglycerol hydrolase or TGH, hydrolyzes TG, DG but not glycerophospholipids. TGH is expressed in the liver, adipose tissue and, to a lesser extent, small intestine, heart and kidney. Ces3/TGH associates with lipid droplets. Attenuation of Ces3/CES1/TGH activity resulted in significantly decreased TG and apolipoprotein B secretion. Conversely, increased expression of CES1/TGH in mice lead to increased apolipoprotein secretion. These results implicated the role of this lipase in the mobilization of preformed TG stores for very-low-density lipoprotein secretion. Interestingly, ablation of Ces3/TGH expression was not accompanied with hepatic TG accumulation. The lack of steatosis was supported by increased hepatic fatty acid oxidation, as well as to decreased TG mobilization in adipose tissue. In addition to the involvement in very-low-density lipoprotein assembly, TGH participates in lipid droplet maturation in hepatocytes. Ces3/TGH deficiency delays the process of lipid transfer from newly synthesized to preformed lipid droplets.
Ces1/Es-x is a mouse carboxylesterase that shares about 76% protein sequence identity with mouse Ces3/TGH, including the residues of the esterase/lipase catalytic triad, neutral lipid binding domain but less homology is apparent in the lid domain, which has been shown to be important for substrate specificity. Ces1/Es-x is only expressed in liver and, in a much lesser extent, in small intestine. Cells stably expressing Ces1/Es-x accumulate significantly less TG in lipid droplets and present with augmented fatty acid β-oxidation.
Ces1/Es-x is responsive to nutritional regulation. Diet supplementation with bile salt cholate and with bile acid-binding resin cholestyramine induced hepatic expression of Ces1/Es-x. Hepatic Ces1/Es-x protein levels are decreased during fasting (a condition when very-low-density lipoprotein secretion is high), while Ces1/Es-x expression in the intestine (low chylomicron secretion) is increased during the same period. In mice refed after a 24 h fast, hepatic expression of Ces1/Es-x increased (attenuated very-low-density lipoprotein secretion), while intestinal expression of Ces1/Es-x decreased (maximum chylomicron secretion). This suggests that Ces1/Es-x may regulate provision of substrates for lipoprotein assembly.
Arylacetamide deacetylase (AADA) shares protein sequence homology with HSL in the active site. AADA is a type II endoplasmic reticulum membrane protein with its active site facing the lumen of the endoplasmic reticulum. In humans, AADA protein is present in the liver and the intestine; however, lower AADA mRNA expression was also observed in adrenal cortex/medulla and pancreas. Cells overexpressing mouse AADA cDNA decreased TG storage and lipoprotein secretion and increased fatty acid β-oxidation. In vitro lipase assay suggested preference for DG, which is in accordance with the homology of this protein with HSL. AADA tissue distribution and activity suggest a role for this enzyme in modulating lipoprotein assembly, but this enticing hypothesis awaits in vivo evidence.
Lysosomal acid lipase is related to gastric lipase and is involved in the degradation of CE and TG derived from endocytosed plasma lipoproteins. Lysosomal acid lipase shows an optimal activity at pH 4–5, which is in agreement with its localization in late endosomes/lysosomes. In humans, lysosomal acid lipase deficiency causes two related diseases, Wolman disease and cholesteryl ester storage disease. Both diseases present with excessive lipid accumulation and hepatomegaly. Animals lacking lysosomal acid lipase die from greatly enlarged livers and spleens and malabsorption due to intestinal infiltration by foam cells. The liver is one of the main organs compromised in these conditions with more than a 30-fold increase of TG and CE levels. Yet, the excessive neutral lipid deposition and augmented cholesterogenesis did not lead to changes in plasma cholesterol and TG levels. During prolonged starvation, lysosomal acid lipase activity may also become important in hydrolysis of cytosolic TG stores to generate fatty acid for β-oxidation through a process termed lipophagy.
Concluding Remarks
Lipolysis is a highly regulated process involving many enzymes and co-factors. The location, specificity and stereoselectivity of lipases determine the metabolic pathways to which the released fatty acids and partial acylglycerols are channeled. That lipases play a crucial role in energy metabolism has become evident from studies in patients with mutations in lipase genes and animal models in which the genes encoding lipolytic enzymes have been deleted. Future studies should be directed at the mechanisms that regulate lipases and their substrate localizations and product channeling.
Recommended Reading
- Miled, N., Canaan, S., Dupuis, L., Roussel, A., Rivière, M., Carrière, F., de Caro, A., Cambillau, C. and Verger, R. Digestive lipases: from three-dimensional structure to physiology. Biochimie, 82, 973-986 (2000) (DOI: 10.1016/S0300-9084(00)01179-2).
- Armand, M. Lipases and lipolysis in the human digestive tract: where do we stand? Curr. Opin. Clin. Nutr. Metab. Care, 10, 156-164 (2007) (DOI: 10.1097/MCO.0b013e3280177687).
- Wang, H. and Eckel, R.H. Lipoprotein lipase: from gene to obesity. Am. J. Physiol. Endocrinol. Metab., 297, E271-E288 (2009) (DOI: 10.1152/ajpendo.90920.2008).
- Doolittle, M.H., Ehrhardt, N. and Peterfy, M. Lipase maturation factor 1: structure and role in lipase folding and assembly. Curr. Opin. Lipidol., 21, 198-203 (2010) (DOI: 10.1097/MOL.0b013e32833854c0).
- Qiu, S., Bergeron, N., Kotite, L., Krauss, R.M., Bensadoun, A. and Havel, R.J. Metabolism of lipoproteins containing apolipoprotein B in hepatic lipase-deficient mice. J. Lipid Res., 39, 1661-1668 (1998).
- Dichek, H.L., Brecht, W., Fan, J., Ji, Z.S., McCormick, S.P., Akeefe, H., Conzo, L., Sanan, D.A., Weisgraber, K.H., Young, S.G., Taylor, J.M. and Mahley, R.W. Overexpression of hepatic lipase in transgenic mice decreases apolipoprotein B-containing and high density lipoproteins. Evidence that hepatic lipase acts as a ligand for lipoprotein uptake. J. Biol. Chem., 273, 1896-1903 (1998) (DOI: 10.1074/jbc.273.4.1896).
- Haemmerle, G., Lass, A., Zimmermann, R., Gorkiewicz, G., Meyer, C., Rozman, J., Heldmaier, G., Maier, R., Theussl, C., Eder, S., Kratky, D., Wagner, E.F., Klingenspor, M., Hoefler, G. and Zechner, R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science, 312, 734-737 (2006) (DOI: 10.1126/science.1123965).
- Ong, K.T., Mashek, M.T., Bu, S.Y., Greenberg, A.S. and Mashek, D.G. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology, 53, 116-26 (2011) (DOI: 10.1002/hep.24006).
- Wu, J.W., Wang, S.P., Alvarez, F., Casavant, S., Gauthier, N., Abed, L., Soni, K.G., Yang, G. and Mitchell, G.A. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology, 54, 122-132 (2011) (DOI: 10.1002/hep.24338).
- Haemmerle, G., Zimmermann, R., Hayn, M., Theussl, C., Waeg, G., Wagner, E., Sattler, W., Magin, T.M., Wagner, E.F. and Zechner, R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem., 277, 4806-4815 (2002) (DOI: 10.1074/jbc.M110355200).
- Taschler, U., Radner, F.P., Heier, C., Schreiber, R., Schweiger, M., Schoiswohl, G., Preiss-Landl, K., Jaeger, D., Reiter, B., Koefeler, H.C., Wojciechowski, J., Theussl, C., Penninger, J.M., Lass, A., Haemmerle, G., Zechner, R. and Zimmermann, R. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J. Biol. Chem., 286, 17467-17477 (2011) (DOI: 10.1074/jbc.M110.215434).
- He, S., McPhaul, C., Li, J.Z., Garuti, R., Kinch, L., Grishin, N.V., Cohen, J.C. and Hobbs, H.H. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J. Biol. Chem., 285, 6706-6715 (2010) (DOI: 10.1074/jbc.M109.064501).
- Basantani, M.K., Sitnick, M.T., Cai, L., Brenner, D.S., Gardner, N.P., Li, J.Z., Schoiswohl, G., Yang, K., Kumari, M., Gross, R.W., Zechner, R. and Kershaw, E.E. Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J. Lipid Res., 52, 318-329 (2011) (DOI: 10.1194/jlr.M011205).
- Chen, W., Chang, B., Li, L. and Chan, L. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology, 52, 1134-1142 (2010) (DOI: 10.1002/hep.23812).
- Dolinsky, V.W., Sipione, S., Lehner, R. and Vance, D.E. The cloning and expression of a murine triacylglycerol hydrolase cDNA and the structure of its corresponding gene. Biochim. Biophys. Acta, 1532, 162-172 (2001) (DOI: 10.1016/S1388-1981(01)00133-0).
- Wang, H., Wei, E., Quiroga, A.D., Sun, X., Touret, N. and Lehner, R. Altered lipid droplet dynamics in hepatocytes lacking triacylglycerol hydrolase expression. Mol. Biol. Cell, 21, 1991-2000 (2010) (DOI: 10.1091/mbc.E09-05-0364).
- Wei, E., Ben Ali, Y., Lyon, J., Wang, H., Nelson, R., Dolinsky, V.W., Dyck, J.R., Mitchell, G., Korbutt, G.S. and Lehner, R. Loss of TGH/Ces3 in mice decreases blood lipids, improves glucose tolerance, and increases energy expenditure. Cell Metab., 11, 183-193 (2010) (DOI: 10.1016/j.cmet.2010.02.005).
- Wei, E., Alam, M., Sun, F., Agellon, L.B., Vance, D.E. and Lehner, R. Apolipoprotein B and triacylglycerol secretion in human triacylglycerol hydrolase transgenic mice. J. Lipid Res., 48, 2597-2606 (2007) (DOI: 10.1194/jlr.M700320-JLR200).
- Dolinsky, V.W., Douglas, D.N., Lehner, R. and Vance, D.E. Regulation of the enzymes of hepatic microsomal triacylglycerol lipolysis and re-esterification by the glucocorticoid dexamethasone. Biochem J., 378, 967-974 (2004) (DOI: 10.1042/BJ20031320).
- Ko, K.W., Erickson, B. and Lehner, R. Es-x/Ces1 prevents triacylglycerol accumulation in McArdle-RH7777 hepatocytes. Biochim. Biophys. Acta, 1791, 1133-1143 (2009) (DOI: 10.1016/j.bbalip.2009.07.006).
- Lo, V., Erickson, B., Thomason-Hughes, M., Ko, K.W., Dolinsky, V.W., Nelson, R. and Lehner, R. Arylacetamide deacetylase attenuates fatty-acid-induced triacylglycerol accumulation in rat hepatoma cells. J. Lipid Res., 51, 368-377 (2010) (DOI: 10.1194/jlr.M000596).
- Du, H., Duanmu, M., Witte, D. and Grabowski, G.A. Targeted disruption of the mouse lysosomal acid lipase gene: long-term survival with massive cholesteryl ester and triglyceride storage. Hum. Mol. Genet., 7, 1347-1354 (1998) (DOI: 10.1093/hmg/7.9.1347).
- Singh, R., Kaushik, S., Wang, Y., Xiang, Y., Novak, I., Komatsu, M., Tanaka, K., Cuervo, A.M. and Czaja, M.J. Autophagy regulates lipid metabolism. Nature, 458, 1131-1135 (2009) (DOI: 10.1038/nature07976).
In This Section
- Glycerophosphate and Acylglycerophosphate Acyltransferases
- Mammalian Diacylglycerol Acyltransferases (DGAT)
- Fatty Acid beta-Oxidation
- Regulation of Lipins and Their Role in Lipid Metabolism
- Phospholipid Biosynthesis
- Phospholipases
- Acylglycerol Lipases (Neutral Lipid Hydrolysis)
- Metabolism and Function of Very-Long-Chain Polyunsaturated Fatty Acids (>C24) in Mammals